
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Case Report(ISSN: 2641-1725) 
Multimodality Correlation in Chronic Budd-Chiari Syndrome in a Young Female with Co-Presentation of Hepatocellular Carcinoma Volume 6 - Issue 1
Bhumika Dua, Rajaram Sharma*, Tapendra Tiwari and Saurabh Goyal
- Pacific institute of medical sciences, Umarda, Udaipur, India
Received: November 19, 2021 Published: December 7, 2021
*Corresponding author: Rajaram Sharma, Pacific Institute of Medical Sciences, Rajasthan, India
DOI: 10.32474/LOJMS.2021.06.000228
Abstract
Budd-Chiari Syndrome (BCS) is an amalgam of pathologies causing hepatic blood outflow obstruction. Long-standing cases of BCS could lead to fibrosis and the development of Hepatocellular Carcinoma (HCC) subsequently. BCS encompasses a wide range of imaging findings ranging from hepatomegaly, ascites, splenomegaly, presence of veno-venous collaterals, thrombus in hepatic vein, compression of IVC, regenerative nodules, nutmeg appearance of the liver and hepatocellular carcinoma in long-standing cases. Our patient presented with complaints of abdominal pain, distension and jaundice. She also had hepatocellular carcinoma, which is a rare co-presentation at such a young age. Triple phase Contrast-enhanced Computerized Tomography (CECT) and Magnetic Resonance Imaging (MRI) proved to be beneficial in supplementing the final diagnosis of BCS and HCC both.
Introduction
Budd-Chiari syndrome (BCS) is described as hepatic venous outflow obstruction. The obstruction may occur at the level of intrahepatic Inferior Vena Cava (IVC) or any of the hepatic veins. It may be primarily due to hypercoagulable or pro-thrombotic state or secondary to obstruction of hepatic veins, inferior vena cava or right atrium. Long-standing BCS may cause sinusoidal congestion, ischaemic injury to hepatocytes, fibrosis, cirrhosis and may eventually lead to Hepatocellular Carcinoma (HCC) [1-4]. The clinical presentation of the patient varies from mild symptoms to acute fulminant hepatic failure, with the majority of patients presenting in the chronic stage [5,6]. The severity of presentation depends on the degree of venous obstruction and formation of veno-venous collaterals [7]. Both BCS and HCC are usually diagnosed by a combination of clinical and imaging features [8,9]. Triple phase Contrast-enhanced Computerized Tomography (CECT) and Magnetic Resonance Imaging (MRI) is the cornerstone for confirmation of the diagnosis of BCS and HCC. There are various options for the treatment of HCC, including Transcatheter Arterial Chemoembolisation (TACE), radiofrequency ablation and surgical resection.
Case Presentation
A 23-year old female presented to our hospital with abdominal pain, distension and yellowish discolouration of the skin for one month. She came to our hospital and was admitted to the medicine ward. On palpation, she had splenomegaly, and tender abdomen and all her blood investigations turned out to be normal. She was advised triple-phase CECT scan of the abdomen and MRI abdomen for further work-up. Our patient was advised CECT triple-phase abdomen and MRI abdomen. The findings favouring the diagnosis of BCS along with HCC were as follows: CT findings: The liver was dysmorphic and had a lobulated surface with altered attenuation. The right lobe of the liver appeared atrophic, with the left and caudate lobe being hypertrophic, giving the appearance of a nutmeg liver. All three hepatic veins were not visualised, and intra-hepatic IVC showed severe narrowing. There was the presence of multiple intra-hepatic veno-venous collaterals. These findings suggested the diagnosis of chronic Budd-Chiari syndrome (Figures 1a&1b). An indistinct geographical lesion was noted in segments II, III and VI. Multiple arterial twigs could be seen arising from the left hepatic artery and supplying the lesion. The lesion was hyper enhancing on arterial phase and had early washout on delayed venous phase, which suggested the diagnosis of HCC. The lesion was directly extending into the left branch of the portal vein. Tumoral thrombus was seen in the left branch of the portal vein, which showed similar morphological features and post-contrast dynamics as of the lesion (Figure1a &1b). There was a non-enhancing partial lumen occluding bland thrombus noted at the confluence of the portal vein. This was the probable cause of the current presentation of the patient (Figure 1c). Multiple well-defined hypodense lesions were also seen in the rest of the liver, which were hypo enhancing on post-contrast images and became isodense to the liver on delayed venous phase. The spleen was moderately enlarged, and the splenic vein was dilated. There was also a mild amount of free fluid in the peritoneal cavity.
Figure 1a and 1b: Axial sections of arterial and delayed venous phases from CECT abdomen at the level of porta, respectively. The right lobe of the liver is atrophied with compensatory hypertrophy of the left and caudate lobe of the liver. The liver shows an ill-defined irregular shaped lesion in segments II, III and VI (white arrow). Few prominent arterial feeders from the left branch of the hepatic artery are supplying the lesion. These arterial branches are also supplying the lesion into the left branch of the portal vein (black arrow). The lesion shows washout of contrast as compared to the remaining liver on the delayed scan, suggestive of HCC.
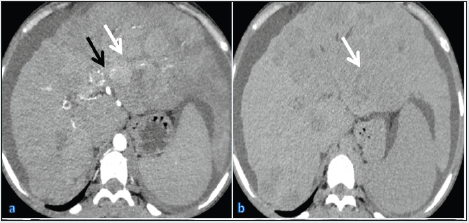
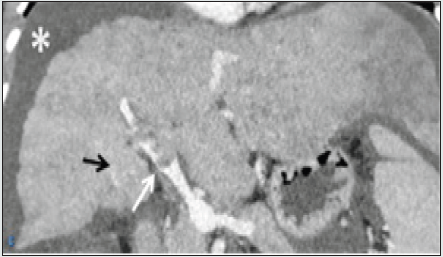
Figure 1c: coronal reformatted section of venous phase from CECT abdomen shows a hypoattenuating partial lumen occluding filling defect in the main portal vein, suggestive of thrombus (white arrow). There are Porto-portal collaterals (black arrow). Mild ascites is also noted (asterisk).
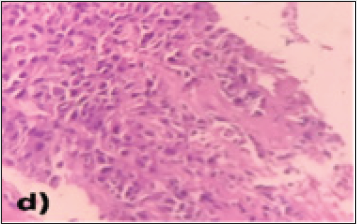
The lung fields also showed multiple, well-defined oval-shaped enhancing soft tissue densities, which were reported as metastatic. MRI findings: The liver showed heterogeneous signal intensity on both T1 and T2 sequences with a nodular outline. The caudate and left lobe of the liver appeared enlarged, and the right lobe was atrophied. In segments II, III and VI of the liver, there was an abnormal intensity lesion which was iso to hypointense on T1 and moderately hyperintense on T2 weighted sequences. The lesion showed extension into the portal vein, consistent with HCC. There were multiple abnormal intensity nodules scattered throughout the liver parenchyma, appearing hyperintense on T1 and hypointense on T2 sequences, suggestive of regenerative nodules (Figures 2a & 2b). The patient underwent an ultrasound-guided biopsy from the lesion, which showed an infiltrating type of HCC. On HPE examination, there were sheets and clusters of atypical hepatocytes showing a high nuclear to chromatin ratio and eosinophilic cytoplasm. The blood vessels also showed inflammatory cells in the walls and extensive areas of surrounding necrosis (Figure 2c & 2d). The patient was referred to the chemotherapy department for further management.
Figure 2a and Figure 2b: Axial sections of MRI of liver on T1 and T2 sequences, respectively. There is an iso to hypointense irregular shaped infiltrative type of lesion observed in segments II, III and VI of the liver, which appears slightly hyperintense on the T2 image (white arrow). The lesion shows extension into the left branch portal vein (black arrow). Mild ascites is also seen (asterisk).
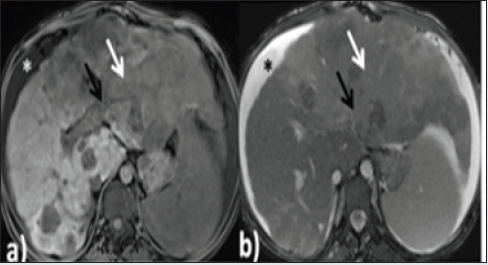
Figure 2c: H&E images, under 10X magnification showing sheets, clusters, lobules and trabeculae of atypical hepatocytes (black arrow) having high N:C ratio, hyperchromatic eccentrically placed nucleus and abundant eosinophilic cytoplasm.
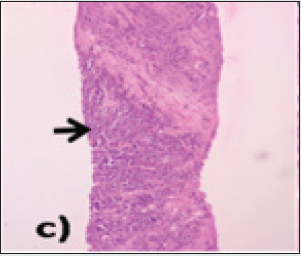
Figure 2d: H&E images under 40X magnification showing intervening stroma with fibro collagenous tissue, inflammatory cell infiltrates and dilated and congested blood vessels with an extensive area of necrosis.
Discussion
BCS is a veno-occlusive disease of the liver due to various causative factors. Venous outflow obstruction may be caused by a thrombus or extrinsic venous compression or due to congenital webs or stenosis [10]. BCS can rarely occur in post-liver transplant patients also. Pregnancy and underlying haematological disorders are also considered risk factors. Hepatocellular Carcinoma (HCC) stands fourth in the list of leading causes of cancer-related death in the world. The incidence of HCC is higher in Asian countries. The etiopathogenesis of HCC is multifactorial; Chronic Hepatitis B Virus (HBV) infection is the most common in India. Long-standing BCS can result in the development of HCC. While most of the patients present with abdominal distension and ascites, some patients also present with gastrointestinal tract bleeding. Cirrhosis occurring in chronic BCS usually has preserved liver functions. The patients are usually young with less severe symptoms [11]. Liver functions, if altered, are only slightly abnormal, and jaundice might be present. The subsequent increase in portal venous pressure in cases of BCS results in splenomegaly (through the splenic vein) and extravasation of transudate in peritoneal space (through SMV); known as ascites. Imaging has a crucial role in making the diagnosis of BCS, especially for patients with insidious onset of symptoms. After the portal vein system shuts down, portosystemic shunts reopen mainly around the esophagus, umbilicus and anal canal, which will take charge; however, these become incompetent in the long term. Liver parenchymal changes and vascular findings should be paid special attention in triple-phase CECT and MRI abdomen for diagnosing BCS as they prove to be a powerful tool in diagnosing BCS in relation to the clinical data. HCC can be differentiated from other liver masses using the triple phase CECT abdomen. On the arterial phase, the lesion shows hyperenhancement on the arterial phase and washout on the delayed venous phase. This feature differentiates HCC from other liver lesions.
References
- Menon KV, Shah V, Kamath PS (2004) The budd-chiari syndrome. N Engl J Med 350: 578-585.
- Aydinli M, Bayraktar Y (2007) Budd-chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol 13: 2693-2696.
- Valla DC (2009) Primary budd-chiari syndrome. J Hepatol 50: 195-203.
- Shin SH, Chung YH, Suh DD, Shin JW, Jang MK, et al. (2004) Characteristic clinical features of hepatocellular carcinoma associated with budd-chiari syndrome: evidence of different carcinogenic process from hepatitis B virus-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol 6: 319-324.
- Ludwig J, Hashimoto E, McGill DB, van Heerden JA (1990) Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd-Chiari syndrome. Mayo Clin Proc 65: 51-55.
- Loomes DE, Chang A, Webber D, Scudamore CH, Yoshida EM (2011) Acute Budd-Chiari syndrome. Can J Gastroenterol 25: 302-303.
- Van Wettere M, Bruno O, Rautou P-E, Vilgrain V, Ronot M (2017) Diagnosis of Budd-Chiari syndrome. Abdom Radiol 43: 1896-1907.
- Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, et al. (2003) Budd-chiari syndrome: A review by an expert panel. J Hepatol 38: 364-371.
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol 35: 421-430.
- Iliescu L, Toma L, Mercan-Stanciu A, Grumeza M, Dodot M, et al. (2019) Budd-Chiari syndrome - various etiologies and imagistic findings. A pictorial review. Med Ultrason 21: 344-348.
- Lin M, Zhang F, Wang Y, Zhang B, Zhang W, et al. (2017) Liver cirrhosis caused by chronic Budd-Chiari syndrome. Medicine (Baltimore). 96: e7425.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




