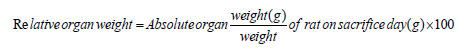Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Evaluation of Antioxidant Activity of The Consciousness Energy Healing Treated Novel Proprietary Formulation after Oral Administration in Male Sprague Dawley Rats Volume 3 - Issue 2
Mahindra Kumar Trivedi1 and Snehasis Jana2*
- 1Trivedi Global, Inc., Henderson, Nevada, USA
- 2Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
Received: March 27, 2019 ; Published:April 03, 2019
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Thane (W), Maharashtra, India
DOI: 10.32474/LOJMS.2019.03.000160
Abstract
The aim of this study was to evaluate the antioxidant and immunomodulatory activity of Consciousness Energy Healing Treated test formulation using antioxidants, hematology and biochemical parameters in male Sprague Dawley rats. A proprietary test herbomineral formulation was prepared and divided into two parts, one was represented as control, while the other was treated with Biofield Energy Treatment by Mr. Mahendra Kumar Trivedi (known as The Trivedi Effect®) and defined as the Biofield Energy Treated sample. The level of malondialdehyde (MDA) was significantly reduced by 11.56%, 30.13%, 24.28%, 38.03%, and 40% in the Biofield Energy Treated test formulation group (G5), Biofield Energy Treated animals per se at day -15 (G6), Biofield Energy Treated test formulation at day -15 (G7), Biofield Energy Treated animals + Biofield Energy Treated test formulation at day -15 (G8) and Biofield Energy Treatment per se to animals plus untreated test formulation (G9), respectively compared to the stress control group (G2). Myeloperoxidase (MPO) was significant reduced by 10.72% in the G5 group compared to the untreated test formulation group (G4). Glutathione (GSH) was significantly increased by 13.69%, 35.71%, and 18.08% in the G4, G5, and G7 groups, respectively than G2. Glutathione peroxidase (GPx) was significantly increased by 97.95%, 97.59%, 98.00%, and 98.14% compared to the G2. Total leukocyte count (TLC) was significantly increased by 19.55%, 34.92%, 45.39%, 41.91%, and 20.25% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G4. Neutrophil count was increased by 13.26% and 11.07% in the G6 and G7 groups, respectively compared to the G4. Triglyceride and very low density lipoprotein (VLDL) were reduced by 24.75% and 24.91%, respectively in the G9 group compared to the G4 group. Creatine kinase - myocardium band (CK-MB) in heart was significantly reduced by 13.91% in the G9 group compared to the G2. Magnesium was increased significantly by 20.33%, 18.42%, 18.05%, 29.32%, 34.59%, and 15.04% in the G4, G5, G6, G7, G8, and G9 groups, respectively compared to the G2. Uric acid was significantly reduced by 34.25% in the G7 group compared to the G4. Besides, feed consumption, body weight, organ to body weight ratio, and histopathology examination did not show any alteration, indicated that Biofield Energy Treated test formulation was found be safe. Overall, data suggested that Biofield Energy Treatment per se and Biofield Energy Treated test formulation have shown significant anti-stress activity and could be utilized in various stress-related disorders like depression and anxiety, acute stress disorder (ASD) and posttraumatic stress disorder (PTSD), sleep difficulties, ADD, ADHD, social avoidance, irritability, including asthma, obesity, diabetes, headaches, gastrointestinal problems, Alzheimer’s disease, accelerated aging, and premature death.
Keywords: Herbomineral formulation; Biofield Energy Healing; The Trivedi Effect®; Antioxidant; Hematology; Biochemistry; Relative organ weight; Histopathology
Introduction
Dysfunction of an immune system leads to various diseases viz. arthritis, ulcerative colitis, asthma, allergy, parasitic diseases, cancer, and infectious diseases. Reduction in the number of the neutrophils and macrophages and phagocytic function is responsible for the suppression of immune system and allows opportunistic pathogens to overwhelm the host to cause secondary infection [1,2]. Antioxidant activity refers to the ability of a compound to delay, inhibit or prevent the oxidation by scavenging the free radicals that leads to reduce oxidative stress. While, oxidative stress has a vital role in the etiology of several diseases and condition such as diabetes, cardiovascular disease, inflammatory condition, cancer, aging, etc. The antioxidant may prevent diseases by scavenging the free radicals, inhibiting lipid peroxidation and by many other mechanisms [3]. In this aspect, authors designed this experiment and formulated the test formulation, which was a combination of trace elements such as zinc chloride, magnesium gluconate hydrate, sodium selenate, Iron (II) sulfate, nanocurcumin, copper chloride, ascorbic acid, and cholecalciferol. Each ingredient of this formulation already been used as nutraceutical supplement [4- 7]. The use of an alternative system of medicine for any types of ailments resulted in an intense area of research and discovery of many herbs and minerals with their potential to cure diseases [8]. The complementary and alternative medicine (CAM) therapy has been increased nowadays for both therapeutic and prophylactic management of various chronic disorders in the human population [9].
Various scientific data reported the positive impact of Biofield Energy Treatment on immune systems [10,11]. The National Center for Complementary and Alternative Medicine (NCCAM) has accepted that by utilizing energy field as Energy Therapies. The Trivedi Effect® - Biofield Energy Healing Therapy already has been popularized to improve the potential health in different group of population benefits. It improved the overall productivity of crops in the field of agriculture [12,13], positive impact on cancer cells [14], altered characteristics features of microbes in the field of microbiology [15, 16], structural, physical, and thermal properties of several metals and ceramics [17,18], improved immunomodulatory activity in in vitro assay [19,20], enhanced skin protective activity [21,22] and improved excellent outcomes of various nutraceutical compounds in the fields of nutraceuticals [23,24] and biotechnology [25,26]. In this study, the impact of Consciousness Energy Healing Treatment (The Trivedi Effect®) on the test formulation was explored in term of immunomodulatory activity, which might be used as a better immunomodulatory function. Therefore, the objective of this experiment was to evaluate the effect of the Biofield Energy Treated and untreated test formulation concerning to antioxidant activity, hematological, biochemical, body weight, feed consumption, and histopathology parameters.
Materials and Methods
Chemicals and Reagents
L-ascorbic acid and sodium selenate were purchased from Alfa-Assar, USA. Zinc chloride and magnesium (II) gluconate were purchased from TCI, Japan. Copper chloride, cholecalciferol (vitamin D3), iron (II) sulphate, and sodium carboxymethyl cellulose were procured from Sigma-Aldrich, USA. Nanocurcumin was obtained from Santa Products Ltd., India. Imipramine hydrochloride used as a positive control was purchased from Abbott Healthcare Pvt. Ltd.
Experimental Animals
The male Sprague Dawley (SD) rats with body weight (200- 265gm) were used in this experiment. The animals were purchased from M/s. Vivo Bio Tech Ltd., Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of eight animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Study Design
A total of nine groups (G) were included i.e. Group 1 (G1) was served as a normal control (i.e. vehicle control), and G2 was served as a stress control (disease control); both the groups were received 0.5% Na-CMC, while G3 group animals received imipramine (20mg/ kg; p.o.). G4 group animals were received untreated test formulation and G5 group received Biofield Energy Treated test formulation at a dose of 624.12 mg/kg. Similarly, G6 animals per se received Biofield Energy Treatment (-15 days); G7 animals received Biofield Energy Treated test formulation (-15 days); G8 group defined as Biofield Energy Treated animals + Biofield Energy Treated test formulation (-15 days) and G9 group denoted as Biofield Energy Treatment per se to animals plus untreated test formulation.
Consciousness Energy Healing Strategies
The test formulation is a combination of eight ingredients such as zinc chloride, sodium selenate, iron (II) sulfate, nanocurcumin, copper chloride, ascorbic acid, cholecalciferol and magnesium (II) gluconate. Each ingredient of the test formulation was divided into two parts. One part of each ingredient was considered as control, where no Biofield Energy Treatment was provided. Another part of each ingredient was received Biofield Energy Treatment by Mr. Mahendra Kumar Trivedi (known as The Trivedi Effect®) under laboratory conditions for ~3 minutes. Besides, three groups of animals were also received Biofield Energy Treatment under laboratory conditions for ~3 minutes. The energy transmission was done without touching the samples or animals. Similarly, the control samples were subjected to “sham” healer under the same laboratory conditions for 5 minutes. The sham healer did not have any knowledge about the Biofield Energy Treatment. After that, the Biofield Energy Treated samples were kept in the similar sealed condition and used as per the study plan. The Biofield Energy Treated animals were also be taken back to experimental room for further proceedings.
Experimental Procedure
Six days after acclimatization, animals were randomized and grouped based on the body weight. Dosing for group G7 and G8 were started on the day -15 till end of the experiment. However, G1 to G6 and G9 animals were dosed from day 1 to till the end of an experiment. Animals (50% of the animals from each group) were kept for overnight fasting on Day 15. However, remaining 50% animals were dosed with respective formulations and kept for fasting on day 16. On the next day, animals were bled, and the samples were subjected to hematology, biochemistry, and electrolytes analysis. After bleeding the animals were sacrificed and all vital organs collected, weight and preserved for histopathology (tissue health) examination. A portion of liver tissues was weighed and homogenized with respective buffer and stored in -80°C for the estimation of various anti-oxidant parameters (LPO, MPO, GSH, and GPx).
Measurement of Antioxidants in Liver Homogenate
Liver homogenate was subjected for the estimation of various antioxidants such as lipid peroxidase (LPO) (Item No. 700870, Cayman chemicals), myeloperoxidase (MPO) (cat No. : K11-0575), glutathione (GSH) (Item No. 703002, Cayman chemicals), glutathione peroxidase (Gaps) (Item No. 703102, Cayman chemicals). The estimation was done as per manufacturer recommended standard procedure.
Hematological and Biochemical Parameters
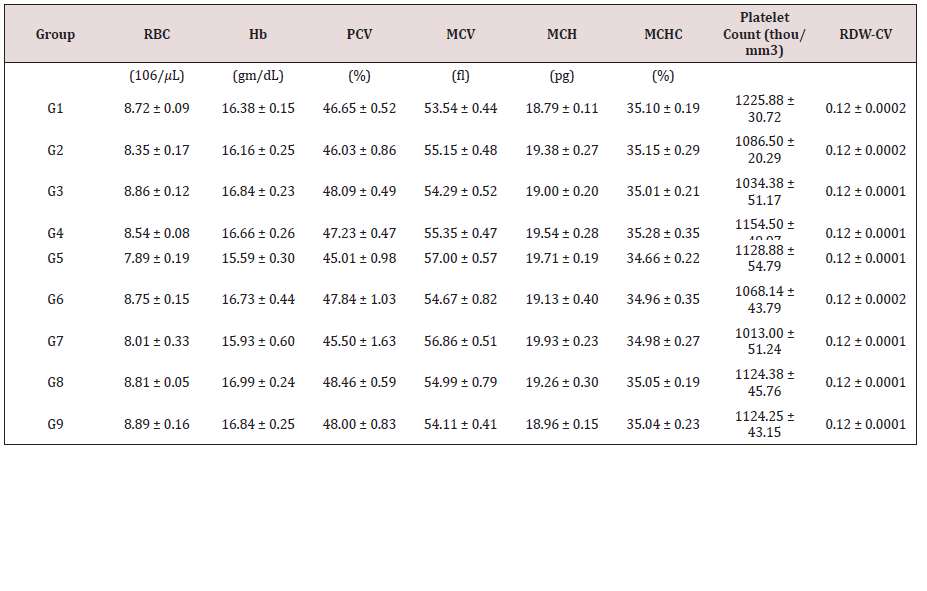
The animals fasted for overnight and blood was withdrawn from retro-orbital plexus under isoflurane anaesthesia. An aliquot of blood sample from each animal was directly subjected for the estimation of hematological parameters viz. red blood count (RBC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and platelets using hematology analyzer (Celta Alpha, Nihon Kho den, Japan). The other aliquot of blood sample was used for the isolation of serum and determined for biochemical parameters viz. magnesium, blood urea nitrogen (BUN), creatinine, uric acid, calcium, phosphorus, potassium, sodium, and chloride ion using biochemical analyzer (MISPA NANO, AGAPEE, India) [27].
Determination of Body Weight and Feed Intake
The body weight and feed intake were measured once daily before the test item administration throughout the experiment. In brief, the daily feed intake was calculated from the difference between the weight of daily feed supply and the left-over feed [28].
Clinical Sign/Symptoms
The clinical signs/symptoms were observed once daily in all the groups as per in-house standard protocol throughout the experiment. Animals those observed as moribund condition or in the state of severe distress were humanely euthanized [29].
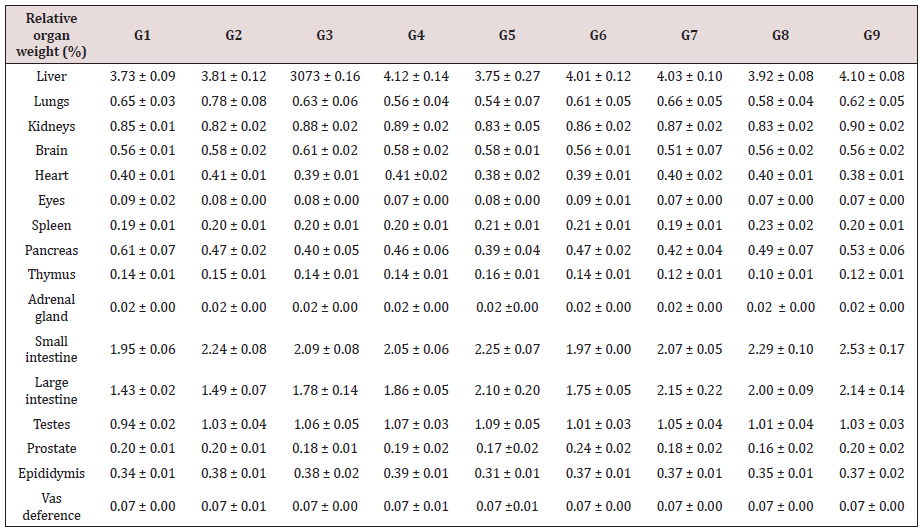
Measurement of Relative Organ Weight and Histopathology
After completion of the study, animals were euthanized by CO2 asphyxiation as per in-house standard protocol. Different organs were excised, weighed, and preserved for histopathological analysis viz. brain, liver, lungs, hearts, kidneys, spleens, eyes, duodenum, jejunum, ileum, caecum, colon, rectum, testis, prostate, pancreas, epididymis, and vas-deference. In brief, the organs were trimmed off of any adherent tissue and fat, as appropriate and were weighed wet as soon as possible to avoid drying. The organ to body weight ratio of individual rat was calculated using absolute weight of every organ with the final body weight. The collected tissues were placed in 10% neutral buffered formalin (NBF) for histopathological examination. Eyes and testis were fixed in Davidson’s fixative and modified Davidson fluid, respectively for 24 hours and followed by 70% alcohol for 48 hours. After that, all the organs were subjected to histopathology as per standard protocol [30,31].
Relative organ weight was calculated using the formula mentioned below-
The data were represented as a mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma Plot (Version 11.0) using one-way ANOVA. Student’s t-test was performed for comparison of the individual treatment group with control. The p≤0.05 was considered as statistically significant.
The effect of the test formulation on the levels of various antioxidant enzymes such as lip peroxides (LPO), myeloperoxidase (MPO), glutathione (GSH), and glutathione peroxides (GPx) is shown in Figures 1A - 1D. The level of malondialdehyde (MDA) was significantly reduced by 11.56%, 30.13%, 24.28%, 38.03%, and 40.00% in the G5, G6, G7, G8, and G9, respectively compared to the stress control group (G2). Further, the level of MDA was significantly decreased by 19.88% and 22.42% in the G8, G9 groups, respectively compared to the untreated test formulation (G4) group Figure 1. The results of myeloperoxidase (MPO) showed a significant reduction by 10.72% in the G5 group compared to the G4 group (Figure 1B). Antioxidant enzyme glutathione (GSH) was significantly increased by 13.69%, 35.71%, and 18.08% in the G4, G5, and G7 groups, respectively compared to the G2 group. Additionally, the level of GSH was significantly increased by 19.37% in the G5 group compared to the G4 group (Figure 1C). Besides, glutathione peroxidase (GPx) was significantly increased by 97.95%, 97.59%, 98.00%, and 98.14% in the G4, G5, G7, & G8 groups, respectively compared to the G2 group. Overall, the Consciousness Energy Healing based test formulation showed better antioxidant activities compared to the untreated test formulation, which might be due to The Trivedi Effect® - Biofield Energy Treatment. The antioxidant enzymes like LPO and others such as MPO, malondialdehyde (MDA), and nitrite are excellent biomarkers for diagnosis of numerous immune diseases [32]. Lodi et al. reported that decreased level of LPO clearly demonstrates the anti-peroxidative activity of Rubbia cordifolia plant extract in renal tissue [33].
Figure 1: The effect of the test formulation on the levels of various antioxidant enzymes. A: Malondialdehyde, B: Myeloperoxidase, C: Glutathione, and D: Glutathione peroxidase (Gaps). G1: Normal control; G2: Stress control; G3-Imipramine; G4-Untreated test formulation; G5-Biofield Energy Treated test formulation; G6: Animals per se received Biofield Energy Treatment (-15 days); G7: Biofield Energy Treated test formulation (-15 days); G8: Biofield Energy Treated animals + Biofield Energy Treated test formulation (-15 days) and G9: Biofield Energy Treatment per se to animals plus untreated test formulation. ***p≤0.001 compared with the G2.
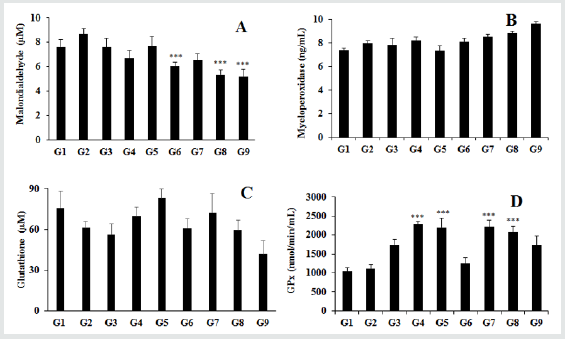
Myeloperoxidase, C: Glutathione, and D: Glutathione peroxidase (GPx). G1: Normal control; G2: Stress control; G3-Imipramine; G4-Untreated test formulation; G5-Biofield Energy Treated test formulation; G6: Animals per se received Biofield Energy Treatment (-15 days); G7: Biofield Energy Treated test formulation (-15 days); G8: Biofield Energy Treated animals + Biofield Energy Treated test formulation (-15 days) and G9: Biofield Energy Treatment per se to animals plus untreated test formulation. ***p≤0.001 compared with the G2.
Determination of Hematological Parameters:
Table 1: Effect of the test formulation on leucocytes (total and differential) counts in male Sprague Dawley rats.
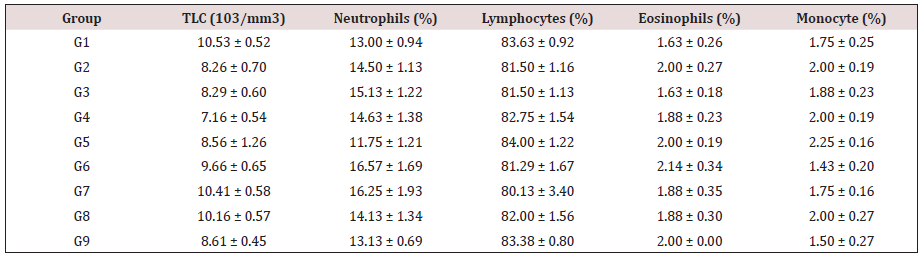
Data are assigned as the mean ± SEM. G: Group, Hb: Hemoglobin; RBC: Red blood count; MCH: Mean corpuscular hemoglobin; PCV: Packed cell volume; MCV: Mean corpuscular volume; RDW-CV: Red cell distribution width and volume. MCHC: Mean corpuscular hemoglobin concentration
The effect of the test formulation on various selected hematological parameters is shown in Table 1. Results showed that total leucocyte count (TLC) was significantly increased by 19.55%, 34.92%, 45.39%, 41.91%, and 20.25% in the G5, G6, G7, G8, and G9 groups, respectively than untreated test formulation (G4). Further, the level of neutrophil was increased by 13.26% and 11.07% in the G6 and G7 groups, respectively compared to the G4 group. Eosinophil was increased by 13.83% in the G6 group compared to the G4 group. Additionally, monocyte was increased by 12.5% in the G5 group compared to the G4 group Table 2.
Evaluation of Biochemical Parameters
Lipid Profile
LDL: Low density lipoprotein; HDL: High density lipoprotein; VLDL: Very low density lipoprotein
Effect of the test formulation on lipid profile is shown in Table 3. The level of total cholesterol was decreased in the G5 group by 5.61% compared to the stress control group (G2) and 8.58% compared to the untreated group (G4). Further, triglyceride was reduced in the G9 group by 21.18% compared to G2 group and 24.75% compared to the G4 group. HDL was increased by 18.49% in the G7 group compared to the G2 group. Level of LDL was reduced by 8.45% in the G5 group compared to the G2 group. The level of VLDL was decreased by 21.28% in the G9 group compared to the G2 group. However, VLDL level was decreased by 6.85%, 7.10% and 24.91% in the G6, G8, and G9 groups, respectively compared to the G4 group.
Hepatic and Cardiac Biomarkers in Serum Sample
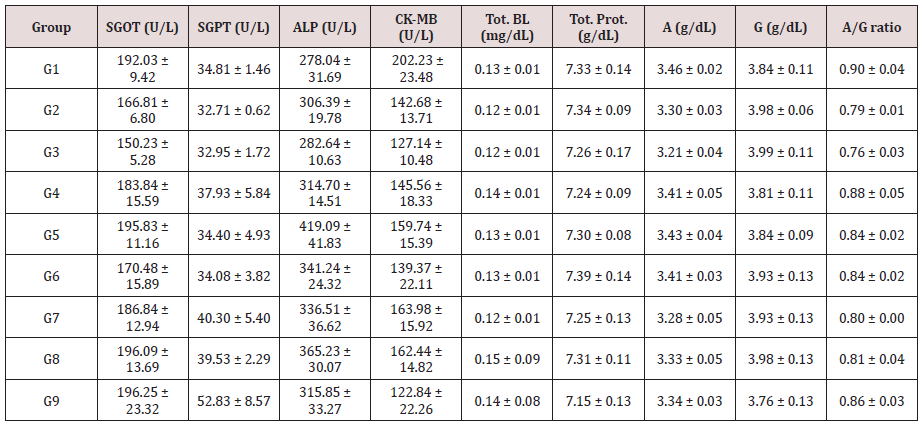
The results of the test formulation on hepatic and cardiac biomarkers is shown in Table 4. The levels of SGOT and SGPT were significantly reduced by 7.27% and 10.15%, respectively in the G6 group compared to the untreated group (G4). The level of creatine kinase myocardial band (CK-MB) was reduced by 13.91% and 15.61% in the G9 group compared to the G2 and G4 groups, respectively. The positive control group (G3) showed 9.94%, 7.75%, and 10.89% reduction of SGOT, ALP, and CK-MB compared to the G2. The level of magnesium was increased significantly by 20.33%, 18.42%, 18.05%, 29.32%, 34.59%, and 15.04% in the G4, G5, G6, G7, G8, and G9, respectively compared to the G2 group. Moreover, level of uric acid was significantly reduced by 23.81% in the G7 compared to the G2; while reduced by 34.25% compared to the G4 group, respectively in the G7 group. The level of calcium was increased by 10.25%, 6.24%, 10.04%, 9.20%, and 6.03% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G2 group. Besides, the content of phosphorous was increased by 11.51%, 13.89%, and 9.58% in the G7, G8, and G9 groups, respectively compared to the G2 group. Allover, a significant increase in the level of serum magnesium, calcium, and phosphorus in the Biofield Energy Treated groups compared to the stress control group (G2). The siginificant outcomes in the Biofield Energy Healing based test formulation might be due to the electromagnetic radiations of the Biofield Energy Healers during energy transmission process.
Table 4: Impact of the test formulation on hepatic and cardiac biomarkers in male rats measured in serum sample.
Clinical Signs/Symptoms
No abnormal clinical signs/symptoms was observed in all the tested animals after treatment with Biofield Energy Treated novel test formulation throughout the experiment at the tested dose Table 5.
Table 5: Assessment of biochemical constituents of the test formulation in male Sprague Dawley rats.
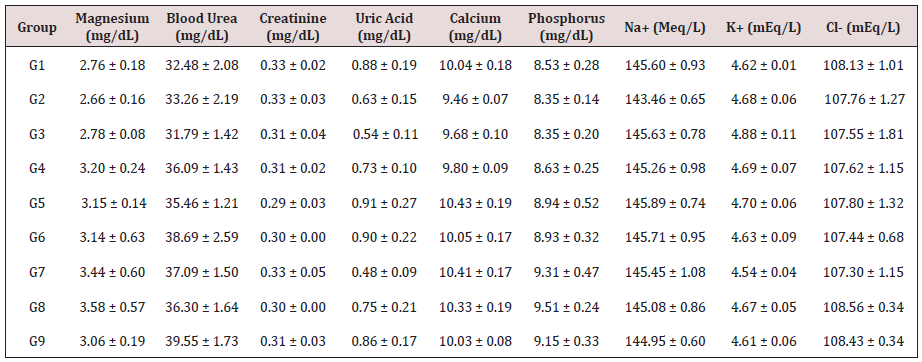
Data are assigned as the mean ± SEM.
Effect of the Test Formulation on Feed Intake, Body Weight, and Organ to Body Weight Ratio
The effect of the Biofield Treated test formulation on animal weight parameters is shown in Table 6. It was found that there was gradual increment of body weight of the animals in all groups and did not found any significant alteration in terms of feed consumption in the tested groups. The outcomes signified that there was no specific changes observed in terms of body weight and feed consumption. Therefore, it is assumed that the Biofield Treated test formulation and Biofield Energy Treatment per se were safe. The organ weight data suggested that the Biofield Energy Treated test formulation (G4) did not produce any signs of organ related toxicity and it was found to be safe with respect to the most of the vital organs toxicity compared to the normal control (G1). The relative organ weight is an indicator for the identification of swelling, atrophy or hypertrophy. The increase organ to body ratio indicates that the test formulation is toxic [34]. However, the experimental results suggest that there was no significant change in most of the vital organs, which ensured that the test formulation was non-toxic to the animals throughout the exposure period.
Assessment of Gross and Histopathological Examination
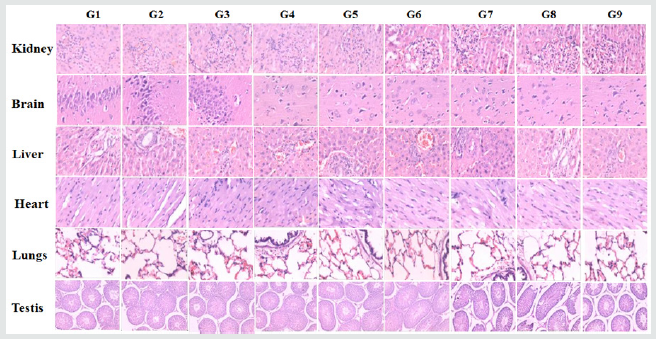
The effect of the Biofield Energy Treatment of the test formulation on histopathology is shown in Figure 2. There were no any distinct differences after microscopic observation of the tested organs. Additionally, histopathological study data also suggest that no treatment-related histopathological findings were reported in all the experimental animals than control. The detailed histopathological images of the selected organs are shown in Figure 2.
Figure 2: Histopathological photomicrograph of major organs. All the tissues were sectioned transversely and stained with hematoxylin (H) and eosin (E).
Conclusion
Based on the study outcomes, it was found that the level of MDA (a free radical) was significantly reduced by 11.56%, 30.13%, 24.28%, 38.03%, and 40% in the G5, G6, G7, G8, and G9, respectively compared to the stress control group (G2). MPO level was significantly reduced by 10.72% in the G5 group compared to the untreated test formulation group (G4). GSH level was significantly increased by 13.69%, 35.71%, and 18.08% in the G4, G5, and G7 groups, respectively than G2 group. GPx level was significantly increased by 97.95%, 97.59%, 98.00%, and 98.14% in the G4, G5, G7, & G8 groups, respectively compared to the G2 group. TLC was significantly increased by 19.55%, 34.92%, 45.39%, 41.91%, and 20.25% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G4 group. TLC was significantly increased by 19.55%, 34.92%, 45.39%, 41.91%, and 20.25% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G4. Neutrophil was increased by 13.26% and 11.07% in the G6 and G7 groups, respectively compared to the G4 group. Further, monocyte was also increased by 12.5% in the G5 group compared to the G4 group. Triglyceride and VLDL were reduced by 24.75% and 24.91%, respectively in the G9 group compared to the G4 group. CK-MB in heart was significantly reduced by 15.61% in the G9 group compared to the G4. Magnesium was increased significantly by 20.33%, 18.42%, 18.05%, 29.32%, 34.59%, and 15.04% in the G4, G5, G6, G7, G8, and G9 groups, respectively compared to the G2. Uric acid was significantly reduced by 34.25% in the G7 group compared to the G4. Further, no treatment-related alteration was observed in the Biofield Treated test formulation concerning the body weight and feed consumption during the experiment. The percentage of an organ to body weight ratio data suggested that the Biofield Energy Treated test formulation was found to be safe concerning the most of the vital organs toxicity.
Overall, the Biofield Energy Treated test formulation showed better antioxidant response without producing any toxicity than untreated test formulation. Therefore, The Trivedi Effect® - Biofield Energy Healing Treatment has shown the significant antioxidant and immunomodulatory activities in male Sprague Dawley rats. It is then anticipatated that the Biofield Treated herbomineral formulation and Consciousness Energy Healing Treatment per se could be the alternative treatment for different disorders like depression and anxiety, acute stress disorder (ASD) and posttraumatic stress disorder (PTSD), sleep difficulties, ADD, ADHD, aging, asthma, obesity, diabetes etc. On the other hand, it can also provide benifical effects for organ transplantation (like kidney, liver, and heart transplantation), and can also be used to treat various autoimmune disorders like Graves’ Disease, Myasthenia Gravis, Addison Disease, Dermatomyositis, Hashimoto Thyroiditis, Pernicious Anemia, Multiple Sclerosis, Aplastic Anemia, Crohn’s Disease, Reactive Arthritis, Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjogren Syndrome, Chronic Fatigue Syndrome Alopecia Areata, Fibromyalgia, Type 1 Diabetes, Psoriasis, Vitiligo, Scleroderma, and Vasculitis, as well as inflammatory disorders such as Ulcerative Colitis, Asthma, Atherosclerosis, Dermatitis, Alzheimer’s Disease, Hepatitis, Diverticulitis, Parkinson’s Disease, Irritable Bowel Syndrome, and stress etc.
Acknowledgement
Authors gratefully acknowledged to Trivedi science, Trivedi testimonials and Trivedi master wellness for their support.
References
- Patwardhan B, Kalbag D, Patki PS, Nagsampagi BA (1990) Search of immunomodulatory agents. Indian Drugs 28: 56-63.
- Fulzele SV, Satturwar PM, Joshi SB, Dorle AK (2003) Study of immunomodulatory activity of Haridradi ghrita in rats. Indian J Pharmacol 35(1): 51-54.
- Pham Huy LA, He H, Pham Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci : IJBS 4(2): 89-96.
- Houston M (2014) The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol 6(2): 38-66.
- Bishop WM, Zubeck HM (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci 2: 147.
- Houston M (2013) Nutrition and nutraceutical supplements for the treatment of hypertension: Part I. J Clin Hypertens (Greenwich) 15(12): 752-757.
- Qureshi NA, Al Bedah AM (2013) Mood disorders and complementary and alternative medicine: A literature review. Neuropsychiatr Dis Treat 9: 639-658.
- Patel P, Basheeruddin Asdaq SM (2010) Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharm J 18(3): 161-165.
- Farhath S, Vijaya PP, Vima M (2013) Immunomodulatory activity of geranial, geranial acetate, gingerol, and eugenol essential oils: Evidence for humoral and cell-mediated responses. Avicenna J Phytomed 3(3): 224-230.
- Lutgendorf SK, Mullen Houser E, Russell D, Degeest K, Jacobson G, et al. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun 24(8): 1231-1240.
- Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, et al. (1996) Massage therapy is associated with enhancement of the immune systems cytotoxic capacity. Int J Neurosci 84(1-4): 205-217.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, Jana S (2015) Analysis of genetic diversity using simple sequence repeat (SSR) markers and growth regulator response in biofield treated cotton (Gossypium hirsutum L.). American Journal of Agriculture and Forestry 3(5): 216-221.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, et al. (2015) Evaluation of vegetative growth parameters in biofield treated bottle gourd (Lagenaria siceraria) and okra (Abelmoschus esculentus). International Journal of Nutrition and Food Sciences 4(6): 688-694.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, Jana S (2015) Antibiogram, biochemical reactions and biotyping of biofield treated Providencia rettgeri. American Journal of Health Research 3(6): 344- 351.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Antimicrobial sensitivity, biochemical characteristics and biotyping of Staphylococcus saprophyticus: An impact of biofield energy treatment. J Women’s Health Care 4(6): 271.
- Trivedi MK, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. (2015) Evaluation of atomic, physical, and thermal properties of bismuth oxide powder: An impact of biofield energy treatment. American Journal of Nano Research and Applications 3(6): 94-98.
- Trivedi MK, Patil S, Nayak G, Jana S, Latiyal O (2015) Influence of biofield treatment on physical, structural and spectral properties of boron nitride. J Material Sci Eng 4: 181.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Balmer AJ, et al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse splenocytes after incubation with biofield treated herbomineral formulation: Effect of biofield energy healing treatment-The Trivedi Effect®. American Journal of Biomedical and Life Sciences 4(6): 87-97.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Ellis MP, et al. (2016) Evaluation of pro-inflammatory cytokines expression in mouse splenocytes after co-incubation with the biofield energy treated formulation: Impact of the Trivedi Effect®. International Journal of Biomedical Science and Engineering 4(5): 40-49.
- Peoples JJ, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Skin rejuvenating effect of consciousness energy healing treatment based herbomineral formulation. American Journal of Plant Biology 2(3): 77- 87.
- Smith DM, Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, Jana S (2017) Skin protective activity of consciousness energy healing treatment based herbomineral formulation. Journal of Food and Nutrition Sciences 5(3): 86-95.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) Chromatographic and spectroscopic characterization of the consciousness energy healing treated Withania somnifera (Ashwagandha) root extract. European Journal of Biophysics 5(2): 38- 47.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Jana S, Mishra RK (2015) Bio-field treatment: An effective strategy to improve the quality of beef extract and meat infusion powder. J Nutr Food Sci 5: 389.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mishra RK, Jana S (2015) Characterization of physical, thermal and spectral properties of biofield treated date palm callus initiation medium. International Journal of Nutrition and Food Sciences 4(6): 660-668.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Singh R, et al. (2015) Physical, Spectroscopic and thermal characterization of biofield treated fish peptone. European Journal of Biophysics 3(6): 51-58.
- Feldman BF, Zinkl JG, Jain VC (2000) Laboratory techniques for avian hematology,” in Schalm’s Veterinary Hematology, (5th Edn) Lippincott Williams & Wilkins, Toronto, Canada.
- Feldman BF, Zinkl JG, Jain VC (2000) Laboratory techniques for avian hematology,” in Schalm’s Veterinary Hematology, (5th Edn) Lippincott Williams & Wilkins, Toronto, Canada.
- OECD, OECD Guideline for Testing of Chemicals (1992) Organization for Economic Cooperation and Development, Paris, France 420.
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, et al. (2007) Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicol Pathol 35(5): 751-755
- Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol Pathol 32(4): 448-466.
- Dhiman M, Coronado YA, Vallejo CK, Petersen JR, Ejilemele A, et al. (2013) Innate immune responses and antioxidant/oxidant imbalance are major determinants of human Chagas disease. PLoS Negl Trop Dis 7(8): e2364
- Lodi S, Sharma V, Kansal L (2011) The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian J Pharmacol 43(4): 441-444.
- Amresh GR, Singh PN, Rao CV (2008) Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116(3): 454-460.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...