
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Case Report(ISSN: 2641-1725) 
Description of the Malaria Coinfection Dengue at Purpose of a Clinical Case Volume 4 - Issue 5
Luis Dulcey1*, Jonathan Pineda1, Hector Moreno1, Jose Sampayo1 and Raimondo Caltagirone2
- 1Residents in Internal Medicine ULA, Venezuela
- 2Specialist in Internal Medicine ULA Mérida, Venezuela
Received: February 07, 2020; Published: February 19, 2020
*Corresponding author: Luis Dulcey, Residents in Internal Medicine ULA, University Hospital of the Andes Mérida, Avenue. 16 of September Service of Internal Medicine Mezzanine Level, Venezuela
DOI: 10.32474/LOJMS.2020.04.000199
Abstract
Introduction: Dengue and malaria coinfection involves different coexisting vectors and hosts maintaining contact with them or traveling to different geographical areas. The first dengue and malaria coinfection report was in 2005 in a French patient, with a history of travel to endemic areas of dengue and malaria, in whom P. falciparum and dengue serotype 3 were diagnosed.
Current disease and background: Male patient of 38 years of age and coming from the Pan-American zone, with no history, who at the beginning of February / 2018 presented generalized arthralgia’s as well as unquantified thermal increases of 3 weeks of evolution, 8 days prior to admission He presents multiple nausea, emetic episodes and abdominal pain, so he goes to our institution.
Physical examination: In stable general conditions. TA 90 / 50 FC104 x’, FR22 x’. Cardiopulmonary Without Alteration, Abdomen generalized pain. Neurological, preserved superior mental functions, preserved cranial nerves, V / V muscle strength in all 4 limbs. It required aggressive management with intravenous fluids. Thickness is confirmed confirming P. vivax infection and serology for Dengue virus type 2 being positive with confirmatory polymerase chain reaction.
Discussion and conclusions of the case: It has been described that the clinical presentation of dengue and malaria coinfection tends to be more severe than in single infections and that it presents with more frequent criteria of severe malaria. On the other hand, it has been observed that the clinical presentation of coinfection is similar to dengue and is imposed on the clinical presentation of malaria.
Keywords: Plasmodium; Coinfection; Dengue; Epidemiology
Introduction
Dengue and malaria coinfection involves different coexisting vectors and hosts maintaining contact with them or traveling to different geographic areas where they are present; [1]It is considered a rare event and is reported infrequently, although several authors believe that there may be sub-registration [2,3]Dengue and malaria coinfection has been reported in different regions of the world with a frequency between 4.1% and 48.5% of all malaria cases and between 4.1% and 77.3% of dengue cases[4,5]. The first report of dengue and malaria coinfection was in 2005 in a French patient, with a history of travel to endemic areas of dengue and malaria, who was diagnosed with P. falciparum (3% parasitic density) and serotype 3 of dengue[6]. Subsequently, cases were reported with other Plasmodium species (P. vivax, P. ovale) [7]with mixed Plasmodium infection (P. vivaxand P. falciparum) [8], with several dengue virus serotypes, cases in women pregnant and in all age groups[9,10]. In India it was observed that the number of cases of coinfection increased during the months August-November, related to the rainy season (Monzón)[11]. It has been described that the clinical presentation of dengue and malaria coinfection tends to be more severe than in single infections [12]and that it presents more frequently severe malaria criteria, the most common jaundice (> 3.0mg / dL), and At least one for severe dengue, the most common vomiting, abdominal pain and bleeding [13]. The duration of the fever is longer in some cases of coinfection and tended to be around 40°C, requiring more hospitalization[14], on the other hand, it has been observed that the clinical presentation of coinfection is similar to dengue and is imposed on the presentation malaria clinic.
Case Presentation
This is a 38-year-old male patient from the Pan-American zone, with no significant pathological history, who at the beginning of February / 2018 presents generalized arthralgias as well as unquantified thermal increases of 3 weeks of evolution, 8 days prior to admission presents nausea accompanied by multiple emetic episodes and abdominal pain so we go to our institution.
Personal history
Denies cardiometabolic, respiratory or other comorbidities.
Family: apparently healthy living parents.
Epidemiological: working in the mines of Bolivar city from
November 2017 to January 2018.
Denies allergic history. Denies surgical history.
Functional and Physical Exam
Functional examination: Patient refers to diffuse abdominal pain of moderate intensity, nausea and multiple emetic episodes. In stable general conditions. TA90 / 50 FC104 x´, FR22 x´. It looks dehydrated with decreased skin turgidity.
Improvement in Penile Curvature
In Gontero study population, the penile extender produced an improvement in penile curvature of clinical interest when compared with that achieved with other commonly used treatment modalities such us intralesional injections. However, results were achieved in a selected population with stable disease, a condition where the existing treatment options are less likely to be effective [9].
Normocephalus without alteration, dry oral mucosa. Mobile neck, without palpable nodes, depressed venous pulse Symmetric, normoexpansible thorax, audible vesicular murmur without aggregates, normophonic, rhythmic heart sounds, with both respected silences, Abdomen generalized pain. Neurological, preserved superior mental functions, preserved cranial nerves, V / V muscle strength in all 4 members. No signs of nuchal stiffness.
Exams and evolution: Enter for the emergency service of our institution before which we are consulted by the referred patient. Multiple studies such as complete hematology are performed, the red series being normal, the platelet count was 34,000.
The leukocyte response was found in 5600 cells at the expense of lymphocytes in 65%.
Renal functionalism within normal with a creatinine of 0.7 mg / dL, the liver profile with a AST and ALT without alterations, total bilirubins at 1.9 mg / dL at the expense of the indirect one at 1.4 mg / dL .
The urine test within normal.
The chest X-Ray showed no abnormalities, the normal cardiothoracic index, no abnormalities in the lung parenchyma.Based on the antecedent of epidemiological type, a battery of exams is sought to look for causal germs of the febrile condition. A thick drop is ordered which performs the Epidemiology service being positive for Plasmodium Vivax.
The referred patient has sustained hypotension, so aggressive management with intravenous fluids was required, which attracted the attention of the treating service in view of the type of Plasmodium type identified in the thick gout. A new thick drop is requested the next day at the request of our service which again reports positivity for Plasmodium Vivax.
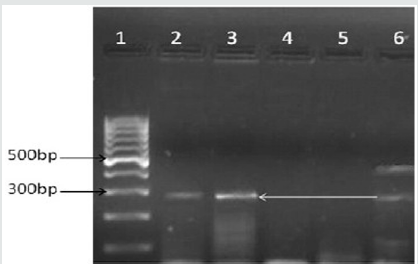
In view of the so torpid clinical evolution, an IgM serology is ordered for Dengue virus which reported positivity, although the multiple cross reactions that occur during the acute episode of Malaria are reported in the literature proceed to order Polymerase chain reaction ( CPR) oriented to identify the presence of Dengue Virus [15]. It is performed in Agarosa Gel with kit (Shanghai ZJ Bio-Tech Company), CPR oriented to identify presence of the viral genome being positive for Dengue Virus serotype 2 (Figure 1).
Figure 1: Polymerase chain reaction in Agarosa gel confirmed presence of viral genome line 1 molecular markers, line 2 Dengue 1 virus, line 3 virus Dengue 2, line 4 virus Dengue 3, line 5 virus Dengue 4, line 6 control.
Management begins with Doxycycline based on publications in this regard [16], on the 4th day there is a decrease in the requirements of intravenous fluids, so the patient goes to the hospital room on the floor. After completing 8 days of hospitalization, handling Chloroquine 25 mg/kg of weight in three days + Primaquine 0.25 mg/kg / day x 14 days, a new thick control drop is performed which was negative, so it was decided to leave and keep the Primaquina on an outpatient basis for 6 more days.
Discussion
The existence of emerging diseases in Venezuela has become
an unprecedented phenomenon. The presence of Dengue and
Malaria as isolated infections entails a high morbidity and mortality
burden, when both infections coexist in the same host, which leads
to a higher mortality [17], the records of said coinfection are scarce,
limited to reports of a single geographical area as in some studies
[18].
In relation to the Dengue of concomitant parasitic infections
[19], the most frequent is co- infection due to malaria (malaria),
of which there are multiple case reports, even complicated with
hemophagocytic lymphohistiociosis [20].
Conclusion
In conclusion, although this is not the first report of dengue and malaria coinfection in the literature, it is the first report of said coinfection in our institution. Malaria infection in Merida was considered eradicated until a few years ago, however most cases are imported from other regions of the country. Having all the information would strengthen the surveillance of dengue and malaria coinfection. We are facing a new national epidemiological environment where multiple uncertainties currently arise which require a human, technical and scientific team at the height of current circumstances, reemerging diseases such as malaria should guide much of our efforts to better understand the pathogenesis and Transmission cycle of each of these diseases separately as well as in the presence of concurrent coinfections.
References
- Shah PD, Mehta TK (2017) Evaluation of concurrent malaria and dengue infections among febrile patients. Indian J Med Microbiol 35(3): 402-405.
- Mørch K, Manoharan A, Chandy S, Chacko N, Alvarez-Uria G, et al. (2017) Acute undifferentiated fever in India: a multicentre study of aetiology and diagnostic accuracy. BMC Infect Dis17(1):665.
- Gadia CLB, Manirakiza A, Tekpa G, Konamna X, Vickos U, et al. (2017) Identification of pathogens for differential diagnosis of fever with jaundice in the Central African Republic: a retrospective assessment, 2008-2010. BMC Infect Dis17(1):735.
- Mueller TC, Siv S, Khim N, Kim S, Fleischmann E, et al. (2014) Acute undi- fferentiated febrile illness in rural Cambodia: A 3-year prospective observational study.Plos one9(4): e95868.
- Assir MZ, Masood MA, Ahmad HI (2014) Concurrent dengue and malaria infection in Lahore, Pakistan during the 2012 dengue outbreak. Int J of Infect Dis18:41-46.
- Charrel RN, Brouqui P, Foucault C, de Lamballerie X (2005) Concurrent dengue and malaria. Emerg Infect Dis11(7):1153-1154.
- Lupi O, Ridolfi F, da-Silva S, Zanini GM, Lavigne A, et al. (2016) Dengue infection as a potential trigger of an imported Plasmodium ovale malaria relapse or a long incubation period in a non-endemic malaria region. IntJ Infect44:20-24.
- Mushtaq MB, Qadri MI, Rashid A (2013) concurrent infection with dengue and malaria: an unusual presentation. Case reports in medicine 2013:2.
- Magalhães B, Alexandre M, Siqueira A, Melo G, Gimaque J, et al. (2012) Clinical profile of concurrent dengue fever and Plasmodium vivax Malaria in the Brazilian Amazon: Case series of 11 hospitalized patients. Am J Trop Med Hyg 87(6):1119-1124.
- Singla N, Arora S, Goel P, Chander J, Huria A (2015) Dengue in pregnancy: an under-reported illness, with special reference to other existing co-infections. Asian Pacific J Trop Med8(3):206-208.
- Hati AK, Bhattacharjee I, Mukherjee H, Bandyopadhayay B, Bandyopadhyay D, et al. (2012) Concurrent dengue and malaria in an area in Kolkata. Asian Pacific Journal of Tropical Medicine5(4):315-317.
- Che Rahim MJ, Mohammad N, Besari AM, Wan Ghazali WS (2017) Severe Plasmodium knowlesi with dengue coinfection. BMJ Case Rep2017.
- Chong SE, Mohamad Zaini RH, Suraiya S, Lee KT, Lim JA, et al (2017) The dangers of accepting a single diagnosis: case report of concurrent Plasmodium knowlesi malaria and dengue infection. Malar J16(1):2.
- Barua A, Gill N (2016) A Comparative Study of Concurrent Dengue and Malaria Infection with their Monoinfection in a Teaching Hospital in Mumbai. J Assoc Physicians India64(8):49-52.
- Kariyawasam R, Lau R, Eshaghi A, Patel SN, Sider D, et al. (2016) Spectrum of Viral Pathogens in Blood of Malaria-Free Ill Travelers Returning to Canada. Emerg Infect Dis22(5):854-61.
- Ahmad S, Dhar M, Mittal G, Bhat NK, Shirazi N, et al. (2016) A comparativehospital-based observational study of mono- and co-infections of malaria, denguevirus and scrub typhus causing acute undifferentiated fever. Eur J Clin Microbiol Infect Dis35(4):705-711.
- Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, et al. (2016) Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J15:47.
- Rao MR, Padhy RN, Das MK (2016) Prevalence of dengue viral and malaria parasitic co- infections in an epidemic district, Angul of Odisha, India: An eco-epidemiological and cross-sectional study for the prospective aspects of public health. J Infect Public Health9(4):421-428.
- Mendonça VR, Andrade BB, Souza LC, Magalhães BM, Mourão MP, et al. (2015) Unravelling the patterns of host immune responses in Plasmodium vivax malaria and dengue co-infection. Malar J14(315).
- Khurram M, Faheem M, Umar M,Muhammad Asad,Iram Khan, et-al. (2015) Hemophagocytic Lymphohistiocytosis Complicating Dengue and Plasmodium vivax Coinfection. Case Reports in Medicine Volume 2015.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




