Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Research Article(ISSN: 2641-6921) 
Toxicological Effect of Household Detergent on Protein Metabolism in Asian Snakehead Fish Volume 5 - Issue 3
Preeti Singh1*, Rakesh Kumar Pandey1 and Balendu Shekher Giri2*
- 1Department of Zoology, Kamla Nehru Institute of Physical and Social Sciences, India
- 2Sustainability Cluster, UPES, India
Received:September 01, 2023; Published: September 11, 2023
*Corresponding author:Preeti Singh, Balendu Shekher Giri, Department of Zoology, Kamla Nehru Institute of Physical and Social Sciences, India, Sustainability Cluster, UPES, Dehradun, Uttarakhand 248007, India
DOI: 10.32474/MAMS.2023.05.000214
Abstract
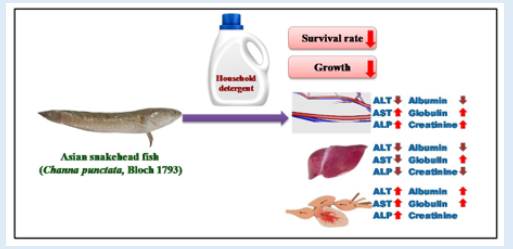
On several enzymatic and protein activities, the toxicities of commercial detergent (Tide), a home washing product, were examined in Asian snakehead fish (Channa punctatus). In a renewal bioassay process, fish were subjected to various detergent doses for 4 days. Variation in protein and others enzymatic levels were ascertained by following the Biuret and Randox techniques, respectively. The 96 h LC50 value was determined using the Finney probit approach. For a 96-hour acute bioassay test, the LC50 result was 0.0060 g/L. Serum aspartate transaminase (AST) in fish subjected to detergent increased significantly (p≤0.05) from 89.55±4.25 to 108.15±2.10, while LFT globulin levels were 0.0024 g/L (2.25 ±0.05) and 0.0048 g/L (2.40±0.10). Across all sublethal values, there was a significant reduction in liver ALP (132±4.50 to 145±2.25), AST (87.10±3.25 to 81.25±2.95, and creatinine (0.32±0.15 to 0.58±0.05). These results postulated that detergent has the possibility of causing negative effects and having a higher influence on the wellness of fishes. Therefore, the contamination of various cleaning agents in an aquatic habitat may be harmful to aquatic bodies and, subsequently, to human health.
Keywords: Fish; Detergent; Biochemical; Toxicity; Asian snakeheads
Introduction
Water contamination has recently become a major issue in India and other developing countries. Apparently, inappropriate chemical disposal, such as the release of detergent wastewater, has led to human and ecological disturbance in household and industrialized areas. When employed with acidic or hard water, detergents can froth. These are cleaning agents derived from synthetic organic compounds [1,2]. Components of detergent such as bleach, filler, foam, soil suspending substances, and other ingredients are formulated to improve the cleaning action. Moreover, surfactants are among the components found in commercial or domestic detergents that are mainly responsible for cleaning activity [3]. These varieties of surfactants and other ingredients are necessary to achieve high solid adsorption coefficients because of their physico-chemical characteristics [4]. Particularly, the molecules of surfactants attach to the floating solids in aquatic bodies and become solid waste along the water's cycle with remediation facilities [5]. In both industrial and household contexts, detergents are frequently employed to wash oil-soiled materials, powerful machinery, automobiles, and other equipment. Due to its frequently employment, detergent is a persistent environmental pollutant [6,7]. Detergents, even those that are biodegradable, are found to have toxic consequences and out of osmoregulation homeostasis in aquatic life, particularly rider present in quantity that are higher than the amount that is utilized for metabolism [8]. Even these foreign substances could be more persistent and mobile in soil and water, making them some of the most ubiquitous outdoor and aquatic pollutants [9]. The gills, liver, kidney, skin, heart, and brain of aquatic bodies were all observed to suffer significant harm as a result of the detergent-containing effluents and discharges [10,12]. Previous research suggested [13,14] that inflexible discharge of effluents or contaminants into an aquatic ecosystem could reduce the concentration of dissolved oxygen, which remained to impair respiration and threaten to cause asphyxiation (which is an expression of unconsciousness or mortality caused by the inability of blood to become properly oxygenated in the lungs) and may eventually lead to organ geometric deterioration, which includes liver dysfunction. Fish wellness is crucial to living beings because fish are essential suppliers of lipids and proteins for both domesticated organisms and mankind [15]. Throughout their life cycle, fish and other aquatic species may be exposed to a wide variety of detergents and pesticides. Fish can absorb various hazardous contaminants through their skin, alimentary ducts, or gills [16,17]. Fish are extremely vulnerable to environmental pollution of the water. Therefore, when pollutants like detergents and pesticides reach the organs of fish, they might considerably harm several physiological and biochemical processes [18]. Therefore, the impact of detergents and pesticides on fish is a major concern. Some earlier studies of household detergents on aquatic bodies were reported. Abbas et al. (2008) reported the severe adverse effects of water contamination on the anatomy of fish [19]. Alterations in the activities of all enzymes in the liver and others such as AST (aspartate transaminase), ALP (alkaline phosphatase), and ALT (alanine transaminase) were observed. Traumatic tissue damage of numerous kinds could result in profound consequences. The presence of these hazardous substances has been reported to interfere with biological and physiological processes; therefore, fish undergo biochemical transformations to preserve homeostasis [20]. Long-term exposure of fish (Labeo rohita) to most toxicants was also investigated and reported to interfere with protein metabolism [21]. There are multiple possibilities for a reduction in total protein in fish that are subjected to toxicant dosage, including a condition of dehydration and a shift in water homeostasis, a disruption in hepatic protein synthesis, or both [22]. Biocatalyst proteins and hormones maintain every mechanism in the organism. A common procedure to diagnose the health of an organ or cell is to examine all types of biocatalyst proteins [23]. As mentioned earlier, fish accepted essential foodstuffs since it contains both proteins and fats for both domesticated animals along with humankind. They are also frequently employed to assess the health of aquatic ecosystems. Changes in physiological function after exposure to pollutants like detergent serve as indicators of aquatic environmental contamination [24]. Since Asian snakehead fish (ASHF) can survive in both well-oxygenated and poorly oxygenated environments and breathe in two modes, these are the most commonly utilized species [25]. In order to evaluate the environmental impacts and stress consequences of manmade causes affecting the physiological state and health of aquatic mammals, fish physiology is an appropriate technique [26]. The findings can provide valuable insights into the potential risks of using household detergents (HHDs) in aquatic environments and their impact on fish populations because of the intimate relationship between fish circulatory systems and their environment [27,28]. The potential for clinical diagnosis of fish physiology provides insight into the effects of external adverse circumstances and hazardous chemicals on the subjected fish.
Experimental
Specimen Collection
Healthy adult Asian snakehead fishes (AASHFs) were obtained at Sultanpur's local market in an unaerated container, with average weights of 27.4±2.50 to 30.0±2.50 g and standard lengths of 14.5±0.5 cm, respectively. The AASHFs were given dry conventional fish meal with 40% crude protein in proportion to 2.5% of their body weight two times a day for the minimum 14-day acclimatization period. The accessible nature of specimens, adaptation to lab settings, ease of management, in-depth understanding of specimen physiology, bio-relevance, and cost-effective considerations were all taken into consideration while choosing AASHF [29].
Measurement of physico-chemical parameters of water
Using a multi-probe potable meter (Hanna Instruments), the water’s physicochemical characteristics, including its temperature (°C), pH, electrical conductivity (EC) (μS/cm), and total dissolved solids (mg/L) were determined. A testing kit from (HI381; Hanna Instruments) was used to measure alkalinity (ppm), and total hardness (TH) (mg/L). Other parameters such as dissolved oxygen (DO) and BOD were determined by methods described by APHA [30].
Bioassay techniques
Five 50-L plastic jars were employed for the bioassay of acute and sub-lethal toxicological experiments. Following an assortment of sensitivity analyses, a stagnant renewal bioassay process [31] was implemented, whereby the test medium was routinely replaced at the same quantity of doses on a daily basis. To establish the definitive amounts appropriate for assessing toxicants (HHD Tide), primary experiments were conducted. Acute toxicity concentrations ranged from 0.032 to 0.008 g/L, with control at 0.00 g/L. Whereas, the three selected concentrations, viz., 12.00, 6.00, and 1.6 mg/L were the fractions (1/5th, 1/10th, and 1/15th, respectively) of the 96 h LC50 value of the detergent. Sub-lethal values were as follows: 0.0048 g/L, 0.0024 g/L, 0.0016 g/L, and 0.00 g/L (control). 10 completely acclimatized samples and a similar number in control experiments were maintained across all investigations.
Acquisition of Blood
In order to prevent or reduce handling stress, three specimens were randomly selected from each of the tanks after 21 days and carefully captured with a hand net. Using a clean piece of cloth, the fluid and water inside the fish's body were dried off. To enable a better grip, the fish's head was draped in a piece of cloth. The vein that runs ventrally down the spinal column was needled with a 24G hypodermic needle-equipped 1 mL sterile plastic syringe to collect blood. Immediately, the blood was placed in EDTA-heparinized vials for further analysis. The vials were further centrifuged at 3000 rpm for 300 s to extract the serum, and they were then kept at -80°C until further studies. The appropriate organs (liver and heart) were swiftly removed from the fish after blood was collected in order to put in order the post-mitochondrial fractions for the planned biochemical and enzymatic studies [32].
Preparation of supernatants (post-mitochondrial fraction)
The heart and liver organs were promptly removed, sterilized in a 1.15% KCl buffer that was ice-cold, wiped on filter paper, and accurately measured. The tissues were then blended and homogenized in four liters of homogenizing buffer (pH 7.4). Then, the resultant supernatant was collected after being centrifuged at 3000 rpm for 10 min from homogenized tissues and kept at -20°C for biochemical examination.
Examine of biochemical parameters
Proper well defined and standard methods were used for evaluating albumin, creatinine, globulin, AST, ALT, ALP, glucose, cholesterol, and total protein. In accordance with albumin's quantitative binding to the bromocresol green (BCG) indicator, it was further quantified. Picric acid and creatinine react in an alkaline solution to produce a colorful complex. This color complex was used to determine the concentration of creatinine at a wavelength of 490 (Agilent, Cary 60 UV-Vis Spectrophotometer). The pyruvate hydrazone was produced by the reaction of ALT and 2,4-dinitrophenyldrazine. AST produces oxaloacetate from 2,4-dinitrophenyldrazine. Both were further monitored with a spectrophotometer at a wavelength between 530 and 550 nm after 5 min in order to determine the ALT and AST concentrations, respectively. Using p-nitrophenyl phosphate as a substrate, ALP analysis was completed to measure the absorbance of the reaction product at 405 nm. The biuret reaction was used to calculate total protein. A violet-colored complex that was formed by combining the amino-acid and peptides into solution having alkaline copper sulphate was determined in comparison to a reagent blank, with measurements collected between 530 and 565 nm [33,34].
Statistical analysis
Probit analysis was applied to examine toxicological concentration data involving quantal response (mortality) for the acute tests [35]. In this investigation, the LC50 and LC0 indices were calculated as lethal and sub-lethal concentrations. Confidence limits were also involved by employing an ANOVA to see whether there were any statistical differences in the composition of plasma and enzymatic proteins. The significance threshold of 0.05 was determined using the Duncan Multiple Ranges Test (DMRT).
Results
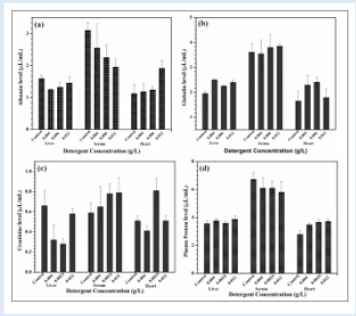
Temperature, pH, DO, conductivity, TDS, alkalinity, TH and BOD all had mean values of 27±0.1°C, 7.58±0.1, 9.20±0.50 mg/L, 185±1.50 µSc/cm, 15 mg/L, 140 mg/L, 150 mg/L and 190.8±0.5 mg/L, respectively. During the bioassay, no negative behavior shifts or fatalities were noticed in the control trial. In AASHFs that had been exposed to HHD, various aberrant behavioral reactions were noticed and stipulated. These reactions included restlessness, repeated jumping and gasping for air, rapid direction changes while moving, relaxing at the bottom, fading skin color, impairment of equilibrium, and a slow start of passivity. The organisms lowered their feeding as well. With higher concentrations, HHD becomes more toxic. The 96-hour LC50 acute toxicity of 0.0060 g/L for household detergent (tide) for fish was observed. Positive linear regression was found in the log of concentration and probit mortality data (Table 1) (Figure 1). AASHF subjected to solution of HHD having sub-lethal concentration of 0.004 g/L had blood ALP levels of 47.5±0.48 that were considerably lower (p≤0.05) than those of the control specimens, which had average values of 52.50±1.25 (Figure 2a). Additionally, there was a considerable reduction in the liver ALP level of exposed AASHFs across all doses, from 132.33±4.50 to 145.40±2.50, compared to the averages of control experiments, which had 154.00±13.00. In contrast to AASHFs in the control tank, exposed AASHFs had not remarkable variations in their cardiac ALP levels across all HHD doses. Although compared to the specimens of control experiment, there was not remarkable difference in the ALT levels of AASHFs's serum, liver, or hearts after ASHs were subjected to the different sub-lethal detergent doses (Figure 2b). There was a substantial rise (p≤0.05) in serum AST values with average values ranging from 109.25±3.50 to 111.25±3.75 across all concentrations of exposed AASHF relative to control values of 89.55±4.25. Additionally, cardiac AST levels significantly increased (p≤0.05) across all concentrations of exposed AASHF, with average values ranging from 85.25±2.0 to 100.25±0.75 in comparison to control, which had median values of 55.25±8.20 (Figure 2c). The liver AST levels of exposed AASHF showed a substantial reduction (p≤0.05) across all sub-lethal detergent doses, with average values varying from 87.10±3.25 to 84.10±10.25 as compared to the control, which had average values of 139.00±3.95 (Table 2). However, there was actually no discernible change in the serum, liver, or heart's total protein content of AASHFs subjected to each of the sub-lethal detergent doses (Figure 3).
Table 1: Probit transformation/analysis of mortality data of Asian Snakehead fish exposed to different concentrations of household detergent (Tide)
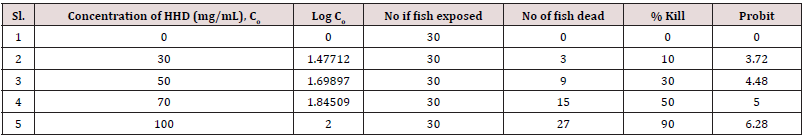
Table 2: Biomarker enzymes (ALT, AST and ALP) in serum of AASHFs at different exposures to HHD and control experiments.
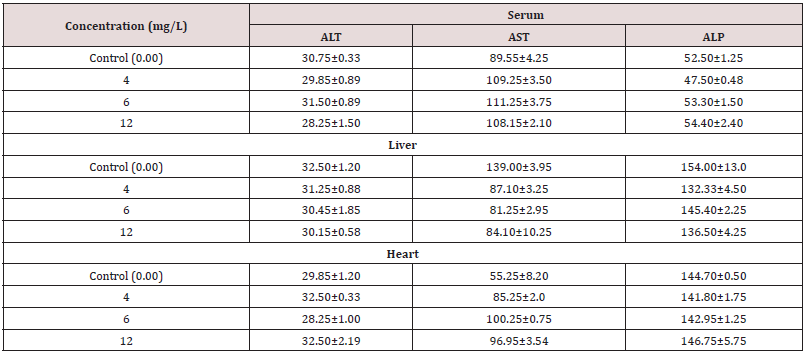
Figure 1: Graph between the percent mortality of fish in 96 h vs. log of household detergent concentration
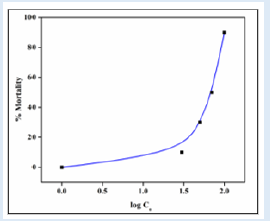
Figure 2: Means and standard deviation of enzyme levels (L) of Asian sneak head fishes exposed to various household detergent concentration Tide and control experiment for 21 days (a) Alanine transaminase (ALT) level, (b) Aspartate aminotransferase (AST) level, (c) alkaline phosphatase (ALP) level.

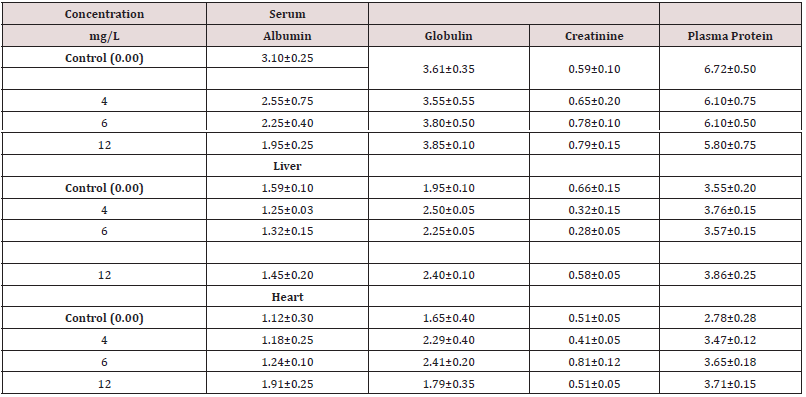
There was a significant decrease in albumin levels in the serum and liver of AASHFs subjected to each concentration of HHD; however, no difference was observed for the heart (Figure 3a). When compared to controls, which had average values of 1.95±0.10, there was a significantly higher amount of liver globulin in the HHD-exposed AASHF at sub-lethal doses of 0.004 g/L and 0.012 g/L with average values of 2.25±0.05 and 2.40±0.10, respectively (Figure 3b). AASHFs subjected to all sub-lethal HHD doses had significantly lower liver creatinine levels (from 0.32±0.05 to 0.58±0.05) (p≤0.05) than controls, which had median values of 0.66± 0.15 (Figure 3c).
Figure 3: Means and standard deviation of total protein levels (L) of asian sneak head fishes exposed to various household detergent concentration Tide and control experiment for 21 days (a) Albumin level, (b) Globulin, (c) Creatinine level, (d) Plasma Protein
Discussion
In order to stabilize the organism under stressful circumstances, the AASHF-body's protective systems undergo modification [36]. Triglycerides and proteins are mobilized by stressed AASHFs to fulfill their enhanced energy requirements as a consequence of increased physical activity, biological transformation, and invasive excretion [37,38]. The impediment of nerve signals between the central nervous system Figure 4 and different beneficiary sites, enzyme dysfunctions that may cause respiration centre depressive symptoms or paralysis, and changes to metabolism or energy routes that lead to a reduction in energy have all been linked to disruptive behavior. The AASHFs in this study exhibited stressed and disruptive behavior Table 3 which is a symptom of respiratory distress and may be caused by the detergent's impact on the gills. This hypothesis and studies on the respiratory impairment of AASHFs exposed to detergent are in line with those of [39-41]. The hyperactivity seen in this study is most likely caused by metabolic abnormalities that lead to energy depletion. It's plausible that animals with higher metabolic rates would need more oxygen and thus engage in more respiratory activity. The lethargy and reduction in equilibrium noticed in the present research may be triggered by the fish's bodies losing energy as a result of being exposed. Additionally, diarrhoea and loss of equilibrium might be signs of difficulties with proper glucose metabolism, which could be caused by enzyme dysfunction [42] reported that the disruption of carbohydrate metabolism causes an energy deficit those results in lethargy and a loss of equilibrium. Organisms that are unable to withstand the toxic substance are put into a state of unconsciousness and eventually die. It was shown that as the detergent dosage was raised; the likelihood of fatalities significantly rose. This is consistent with the findings of [43].
Table 3: Biomarker enzymes (Albumin, globulin, creatinine, and plasma protein) in serum, liver and heart of AASHFs at different exposures to HHD and control experiments.
The presence of the non-plasma-specific enzymes ALT, AST, and ALP in the blood may provide particular information regarding organ failure since they are localized in the tissue cells of the liver, heart, gills, kidneys, muscles, and other organs [44]. This study's observation of lower blood and liver AST levels in AASHFs that exposed to HHD indicates lower demands for energy, amino acids, and metabolic pathways [45] found that in Cyprinus carpio exposed to diazinone, the reduction in ALT and AST activity in the detergent-exposed fish validates these findings [46] observed that diazinone significantly reduced the blood levels of the enzymes APT and acid phophatase in Clarias gariepinus, although AST and ALT were similar in fish subjected to control. The parenchymatous tissue and skeletal muscles remain unharmed; therefore, a reduction in the transaminases indicates there was not any tissue destruction; however, there was a reduction in the rate of amine group exchange, which ultimately lowers the rate of protein and carbohydrate biosynthesis in AASHFs. The reduction in ALP levels in the liver of HHD-exposed AASHFs may be associated with decreased glycogen formation brought on by decreased metabolic requirements as well as electrolytic instability brought on by excessive tissue dehydration. The studies of Shaffi (1975) on the effects of starvation on tissues and serum ALP of Heteropneustes auriculata are consistent with this [47]. Albumin and globulin, two plasma proteins, play a crucial role in the transport of nutrients from a portion of the body to elsewhere. Because proteins are involved in enzymes, hormones, and antibiotics, as well as osmotic pressure control and regulating acid-base homeostasis, they have significant diagnostic importance [48]. The amounts of total protein may rise, fall, or show no discernible pattern. In this investigation, there was no discernible difference between the HHD-exposed AASHFs and the control group in terms of protein levels. The concentrations used and, most likely, the exposure duration may help to explain this. The most prevalent protein in plasma is albumin. It is produced in the liver at a pace influenced by protein consumption and controlled by plasma albumin levels. A reliable sign of the health of the glomerular and other membranes is albumin. Its primary roles include acting as a source of endogenous amino acids and transporting and storing a wide range of ligands to maintain plasma oncotic pressure [49]. Additionally, proteins make up globulin, some of which the liver and others the immune system produce. The subunits of globulin are thought to be the source of practically all the immunologically active proteins in the blood [50]. In general, it is believed that higher innate immune responses are linked to increases in globulin levels in fish [51). The study's findings reveal a rise in the liver globulin levels of AASHFs subjected to HHD, which is a sign of the AASHF's immune system's reaction to the toxin. An indicator of destruction of the liver, renal acid, and muscle purine metabolism is creatinine. In the present investigation, there was also discernible variation in creatinine levels. Tilapia zillii treated with aluminium had a higher creatinine level, according to Additionally, it was said that glomerular insufficiency, an increase in the deterioration of muscle tissue, or a problem with glucose metabolism might all lead to a rise in creatinine levels.
Conclusion
Apparently, sub-lethal detergent doses can cause an assortment of toxicological consequences in AASHF (Channa punctatus) in the form of enzymatic degradation. It may be deduced that the addition of detergent in aquatic environments might cause organ and enzymatic destruction, which could render all living things in a polluted environment susceptible to health issues and finally cause mortality. The activities of enzymes can therefore be effectively employed to assess the impact of detergent on the physiology of fish under sub-lethal conditions prior to unanticipated mortality. Any aberrant alterations in the physiology of aquatic species can be easily recognized, and corrective action can be taken before the start of epidemics if comprehensive environmental quality assessment is made essential and continual water quality monitoring is carried out.
Statements and Declarations
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Okpokwasili GC, Nwabuzor CN (1988) Primary biodegradation of anionic surfactants in laundry detergents. Chemosphere 17: 2175-2182.
- Esenowo IK, Ugwumba OA (2010) Growth Response of Catfish (Clarias gariepinus) Exposed to Water Soluble Fraction of detergent and diesel oil, Environmental Research Journal 4(4): 298-301.
- Yu Y, Zhao J, Baylym AE (2008) Development of Surfactants and Builders in Detergent Formulations, Chinese Journal of Chemical Engineering 16(4): 517-527.
- Linke D (2009) Detergents An Overview. in: Methods in Enzymology, (Eds.) R.R. Burgess, M.P. Deutscher Academic Press pp 603-617.
- Hammerton C (1955) Observations on the decay of synthetic anionic detergents in natural waters, Journal of Applied Chemistry 5(9): 517-524.
- Warne MSJ, Schifko AD (1999) Toxicity of Laundry Detergent Components to a Freshwater Cladoceran and Their Contribution to Detergent Toxicity. Ecotoxicology and Environmental Safety 44(2): 196-206.
- Eniola KIT, Olayemi AB (2000) Some Aspect Bacterial- Detergents interaction in freshwater environment, Bioscience Research Communication 14: 645-649.
- Ezemonge LN, Ogeleka DF, Okieimon FE (2007) Acute Toxicity of Industrial Detergent (Neatex) and Corrosion Inhibitor (Norust CR486) to Early Stages of Cichlids (Tilapia guineensis), Chemistry and Ecology 23: 131-138.
- El-Nahhal Y (2018) Toxicity of some aquatic pollutants to fish, Environmental Monitoring and Assessment 190(8): 449.
- Singh AK, Chandra R (2019) Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards, Aquatic Toxicology 21: 202-216.
- Wang JQ, Hussain R, Ghaffar A, Afzal, G, Saad AQ, et al. (2022) Clinicohematological, Mutagenic, and Oxidative Stress Induced by Pendimethalin in Freshwater Fish Bighead Carp (Hypophthalmichthys nobilis), Oxidative Medicine and Cellular Longevity 28: 2093822.
- Padrilah SN, Sabullah MK, Shukor M, Yasid NA, Shamaan NA, et al. (2018) Toxicity effects of fish histopathology on copper accumulation, Pertanika Journal of Tropical Agricultural Science 1(2): 519–540.
- Doust JL, Schmidt M, Doust LL (1994) Biological assessment of aquatic pollution: a review, with emphasis on plants as biomonitors, Biological Reviews 69(2): 147-186.
- Amoatey P, Baawain MS (2019) Effects of pollution on freshwater aquatic organisms, Water Environment Research 91(10): 1272-1287.
- Tilami SK, Sampels S (2018) Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals, Reviews in Fisheries Science & Aquaculture 26(2): 243-253.
- Schlenk D (2005) Pesticide biotransformation in fish. in: Biochemistry and Molecular Biology of Fishes, (Eds.) T.P. Mommsen TW Moon 6: 171-190.
- Banaee M, Ahmadi K (2011) Sub-lethal toxicity impacts of endosulfan on somebiochemical parameters of the freshwater crayfish (Astacus leptodactylus), Research Journal of Environmental Sciences 5(11): 827-835.
- Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2013) Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon, Fish Physiology and Biochemistry 39(3): 489-501.
- Abbas HHH, Mahmood HM (2008) The Toxicological Effect of Water Pollution on the Nile Tilapia Fish (Oreochromise niloticus) collected from four sites along the River Nile, Journal of the Egyptian Veterinary Medical Association 63: 307-323.
- Abbas HHH, Mahmood HM (2004) Heamatology and Biochemical Changes in Oreochromise aureus and Clarias gariepinus exposed to mixture of copper and lead salts, Egyptian Journal of Basic and Applied Physiology 3: 89-106.
- Das BK, Mukherjee SC (2000) Chronic toxic effects of quinalphos on some biochemical parameters in Labeo rohita (Ham.) Toxicology Letters 114(1-3): 11-18.
- Manoj K (1999) Mercury copper and cadmium induced changes in the total protein levels of muscle tissue of edible estuarine fish (Boleopthalmus dessumuri), Journal of Environmental Biology 20: 231-234.
- Tabassum H, Dawood AQ, Sharma P, Khan J, Raisuddin S, et al. (2016) Multi-organ toxicological impact of fungicide propiconazole on biochemical and histological profile of freshwater fish Channa punctata Bloch, Ecological Indicators 63: 359-365.
- Reddy PB, Waskale K (2013) Using histopathology of fish as a protocol in the assessment of aquatic pollution. Journal of Environmental Research and Development 8(2): 371-375.
- Datta SN, Kaur VI, Dhawan A, Jassal G (2013) Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. SpringerPlus 2(1): 436.
- Alkahem HF, Ahmed Z, Al-Akel AS, Shansi MJK (1998) Toxicity bioassay and changes in haematological parameters of Oreochromis niloticus induced by trichloroform Arab Gulf Journal of Scientific Research 16: 581-585.
- Celik ES (2004) Blood chemistry (electrolytes lipoproteins and enzymes) values of black scorpion fish (Scorpaena porous) in the Dardanelles, Turkish Journal of Biology 4: 716-719.
- Cech JJ, Bartholow SD, Young PS, Hopkins TE (1996) Striped bass exercise and handling stress in fresh water: Physiological responses to recovery environment. Transactions of the American Fisheries Society 125: 308-320.
- Solbe JF (1995) Freshwater In: Handbook of Ecotoxicology (Eds) Peter Collins. Blackwell Science limited. Osneymeed OX 20EL pp. 683.
- Rice EW, Bridgewater L (2012) Association APH., Standard methods for the examination of water and wastewater American public health association Washington DC, USA 10.
- Mustapha DS, Bawa-Allah KA (2020) Differential toxicities of anionic and nonionic surfactants in fish, Environmental Science and Pollution Research 27(14): 16754-16762.
- Pedroso GL, Hammes TO, Escobar TD, Fracasso LB, Forgiarini LF, et al. (2012) Blood collection for biochemical analysis in adult zebrafish, Journal of Visualized Experiments 63: e3865.
- Reitman S, Frankel S (1957) A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. American Journal of Clinical Pathology 28: 56-63.
- Brandenberger H Hanson R (1953) A spectrophotometric method for estimation of acid and alkaline phosphatases, Helvetica chimica acta 36: 900-906.
- Douglas MT, Chanter DO, Pell IB, Burney GM (1986) A proposal for the reduction of animal numbers required for the acute toxicity to fish test (LC50 determination) Aquatic Toxicology 8(4): 243-249.
- Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progenyOregon Agricultural Experiment Station Technical Report Number 11578. in: Reproductive Biotechnology in Finfish Aquaculture, (Eds.) Lee CS, Donaldson EM, Elsevier. Amsterdam p. 3-24.
- Arjona FJ, Vargas-Chacoff L, Ruiz-Jarabo I, Gonçalves O, Pásco I, et al. (2009) Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 287(3): 419-426.
- Adineh H, Naderi M, Khademi H, Amidi M, Harsij M (2019) Biofloc technology improves growth, innate immune responses, oxidative status, and resistance to acute stress in common carp (Cyprinus carpio) under high stocking density. Fish & Shellfish Immunology 95: 440-448.
- Adewoye SO, Fawole OO, Owolabi OD, Omotosho JS (2005) Toxicity of cassava wastewater effluents of African Catfish: Clarias gariepinus. Ethiopian Journal of Science 28 189-194.
- Ayoola SO (2008) Toxicity of glyphosate herbicide on Nile tilapia (Oreochromis niloticus) juvenile. Agricultural Research 3: 825-834.
- Ogundiran MA, Fawole OO, Adewoye SO, Ayandiran TA (2009) Pathologic Lesions in the Gills structures of Clarias gariepinus on exposure to sub-lethal concentrations of soap and detergent effluents. The Journal of Cell and Animal Biology 3: 078-082.
- Anderson T, Forlin L, Hardig J, Larsson A (1988) Physiological disturbances in fish living in Coastal water polluted with bleached Kraft pulp mill effluents. Canadian Journal of Fisheries and Aquatic Sciences 45: 1525-1536.
- Gouda AMR, Hagras Okbah MA, El-Gammal MI (2022) Influence of the Linear Alkylbenzene Sulfonate (LAS) on hematological and biochemical parameters of Nile Tilapia, Oreochromis niloticus. Saudi Journal of Biological Sciences 29(2): 1006-1013.
- Gabriel U, George ADI (2005) Plama enzymes in Clarias gariepinus exposed to chronic levels of roundup (glyphosate). Environmental Ecology 25: 271-276.
- Luskova V, Svoboda M, Kolarova J (2002) The effects of diazinon on blood plasma biochemistry in carp (Cyprinus carpio). Acta Veterinaria Brno 71: 117-123.
- Adedeji OB, Adeyemo OK, Agbede SA (2009) Effects of diazinon on blood parameters in the African catfish (Clarias gariepinus). African Journal of Biotechnology 8: 3940-3946.
- Shaffi SA (1979) Effects of starvation on tissue and serum gluconeogenic enzymes, alkaline phosphates and tissue glucogen in freshwater catfish Heteropnuestes fossilis (Bloch). Acta physiologica Academiae Scientiarum Hungaricae 53: 501-505.
- Hadi A, Shoker A, Alwan S (2009) Effect of aluminium on the biochemical parameters of freshwater fish Tilapia zillii. Journal of Applied Sciences 3: 33-41.
- Andreeva AM (2019) The Strategies of Organization of the Fish Plasma Proteome: with and without Albumin. Russian Journal of Marine Biology 45(4): 263-274.
- Jha AG, Mekkawyl AA, Verreth J, Kirschbaum F (2007) Effects of lead nitrate on the activity of metabolic enzymes during early developmental stages of the African Catfish. Clarias gariepinus (Burchell, 1822). Fish Physiology and Biochemistry 33: 1-13.
- Sastry KV, Gupta PK (1980) Alterations in the activities of a few dehydrogenases in the digestive system of two teleost fishes exposed to lead nitrate Ecotoxicology and Environmental Safety 4: 232-239.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...