Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Research Article(ISSN: 2641-6921) 
The Mechanisms of Sulphate Attack in Concrete – A Review Volume 5 - Issue 2
Hafiz Muhammad Nadir and Ash Ahmed*
- Civil Engineering Group, School of Built Environment & Engineering, Leeds Beckett University, Civic Quarter, Northern Terrace Leeds, UK
Received:October 14, 2022; Published: October 21, 2022
*Corresponding author:Ash Ahmed, Civil Engineering Group, School of Built Environment & Engineering, Leeds Beckett University, Civic Quarter, Northern Terrace Leeds, UK
DOI: 10.32474/MAMS.2022.05.000206
Abstract
The exposure of engineering structures to complex chemical hazards in omnifarious geographical/ environmental locations and emission of greenhouse gases from manufacturing and usage of cement have encouraged researchers to explore the chemical synthesis taking place in the blending of different raw materials, formation of complex compounds, hydration of cement concrete and reactions taking place during internal/ external sulphate attacks. This study has carried out an in-depth elucidation of the contemporary research to understand better the raw material composition of cement-like calcareous and argillaceous minerals, hydration process, the reaction of complex compounds to create cement paste, kinetics associated with the formation of ettringite, monosulphate aluminate ferrate hydrates, exchange of cations and anions between reactive metals hydroxide, hydrates, sulphates and their impacts on long term sustainability properties of concrete. An endeavour has been made to explore the use of lime and pozzolans derived from industrial, agricultural and natural resources. The microstructural studies were examined, which augmented the research findings that the development of cracks/ failure in concrete is attributed to the formation of ettringite, gypsum, brucite, M-S-H gel, thaumasite, portlandite, expansive silica hydroxide gel and carbonation of metal hydroxides due to internal/ external sulphate attacks on samples having more water-cement ratio and exposed to more concentrated magnesium/ sodium sulphate solutions.
Keywords: Cement; hydration; sulphate attack; supplementary cementitious materials; microstructural studies
Introduction
The human’s desire to conquer oceans, rivers, plains, mountains and deserts has led to the construction of diverse infrastructure in different environments, thus giving rise not only to disturbance to the natural environment but equally causing hazards to infrastructure from the environment too in the form of deterioration/ depletion due to ageing, spalling, thawing, corrosion, erosion, temperature variation, chemicals attacks, ingress of moisture/ acidic/ alkaline solutions and different atmospheric conditions [1]. Historically, the building materials, like stone, clay, lime, pozzolanic binders, and modern-day cement-based ingredients, have been on the construction inventory for various construction requirements. One of the most widely used materials in modern construction is concrete which is preferred due to its mechanical properties and ease of use [2,3]. The variation in the performance of cement concrete in different climatic conditions is its vulnerability against external/ internal chemical attacks, especially sulphate attacks, over an extended exposure period [2-8]. These phenomenal concrete vulnerabilities have encouraged researchers to study the chemical synthesis igniting the sulphate attack and to formulate composite sustainable materials [9,10]. This paper reviews contemporary research conducted on cement composition, chemical reactions, hydration process, chemo-mechanical kinetics and microstructural synthesis during sulphate attack.
Literature Review on the use of cement, lime and SCMs
Lime
The quicklime CaO and slaked lime Ca(OH)2 are considered among the oldest construction materials which have been in use for thousands of years in construction since the inception of great civilisations like Romans, Greek and Egyptians etc. The naturally occurring calcareous rocks (limestone) containing CaCO3 are burnt to produce pure lime, called the calcination process and are considered the major emitter of direct CO2 gas [11]. The lime is then used as hydrated lime Ca(OH)2 by slaking the CaO with water as internal/ external masonry, mortaring, faced work and paints etc. [12]. The natural hydraulic lime is manufactured under BS EN 459-1:2015 and broadly available in the industry as NHL2, NHL3.5 and NHL5 for use as lime mortars, plasters, and renders with 2-5 MPa strength. The non-hydraulic lime, also known as soft/ slow setting lime putty, is the third type of industrial lime used in construction [12,13]. When used as construction material, the hydrated slaked lime Ca(OH)2 absorbs CO2 and slowly sets to create hardened CaCO3, thus completing a lime cycle as illustrated in the following equations 1-3 [10-14]

Ordinary Portland Cement (OPC)
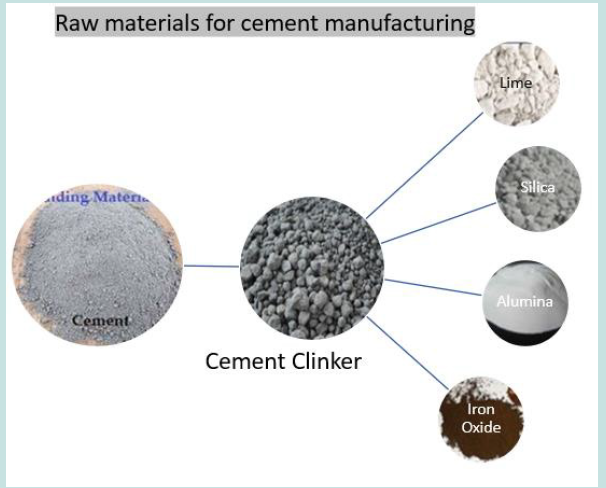
The calcareous rock (lime) is an essential ingredient of cement manufacturing (around 62%), where it is mixed with argillaceous minerals containing clay/ aluminosilicates (about 27%) in the kiln over 1600 Co to produce clinker, which is then ground to less than 2 µm fine particles. Magnesia (about 2%), alkalis like soda and potash (around 1%), gypsum (about 4%) and iron oxide (about 3%) are also found in ordinary Portland cement [15-18]. Cement is said to be invented by Egyptians and has since been in use in different forms/ compositions by different nations/civilisations, including Babylonians, Assyrians, Romans, Greeks, Gothics, Chinese, Russians, Europeans and Americans but OPC was first produced in its present form in England using Portland claystone from the Isle of Portland by Joseph Aspdin in 1822, which was further refined by his son William Aspdin in 1840 as calcium silicates and further improved by Isaac Charles Johnson in 1850s. The present-day cement is primarily divided into non-hydraulic and hydraulic cement, e.g., OPC [18-22]. R.H. Bogue first identified the broadly occurring chemical reaction in 1960 by identifying four main components in cement and are known as Bogue’s compounds which include alite (C3S tricalcium silicate 3CaO.SiO2), belite (C2A dicalcium silicate 2CaO.SiO2), celite (C3A tricalcium aluminate 3CaO.Al2O3) and felite (C4AF tetra calcium alumina ferrite 4CaO.Al2O3.Fe2O3). Alite exhibits medium setting time, is highly exothermic, and gives early strength in the first phase of hydration. Belite demonstrates low setting time, is less exothermic, gives ultimate strength in the second hydration phase, and provides defence against chemical attacks. Celite causes flash setting, is less exothermic, does not contribute to the strength and induce vulnerability for internal/ external sulphate attacks. Felite exhibits slow setting time, is less exothermic, does not contribute to strength, and helps prevent sulphate attacks [23-26]. Bogue’s compound composition in cement is shown in (Figures 1-3) and (Table 1).
The Cement Concrete Hydration Process
The dry hydraulic cement OPC does not constitute any strength and needs water for its hydration and initiation of chemical reaction, leading to its setting/ hardening and gaining of strength over a curing time of around 3 - 90 days. The hydration process is exothermic and starts quickly by mixing with water. Lime and silica are responsible for the early setting of cement concrete and producing strength [27]. Alite (C3S tricalcium silicate 3CaO.SiO2) is the compound which reacts at first instance with water and produces calcium silicate hydrate, also known as C-S-H gel (3CaO.2SiO2.3H2O) responsible for the strength of concrete. The excess quantity of portlandite or calcium hydroxide Ca(OH)2 is responsible for the reduction in strength of concrete and long-term adverse effects due to its reaction with sulphates to form gypsum. C3S produces 61% C-S-H gel and 39% Ca(OH)2 giving 60% of overall concrete strength and releasing 500 Joules/gram of heat of hydration [27-30] (equation 4) [28].

Belite (C2S dicalcium silicate 2CaO.SiO2) produces 82% C-S-H gel and 18% Ca(OH)2 in the second phase of hydration and is generally responsible for prolonged development of strength in later curing time, measuring up to 30% of overall concrete strength, releasing 260 Joules/gram of heat of hydration (equation 5) [28].

Considering the quantity of formation of C-S-H gel and Ca(OH)2 by C3S and C2S, it can be inferred that C3S produces more strength but more vulnerability too in contrast to C2S, which contributes lesser strength and lesser vulnerability too due to the percentage formation of Ca(OH)2 respectively. Therefore, the percentage components of C3S and C2S in cement are critical and generally comprise 70-80% of the overall hydration process. Increasing C3S will increase setting time and strength but decrease defence against chemical attacks. Increased C2S will increase delayed strength, delay setting time and improve vulnerability against sulphate attack. Celite (C3A tricalcium aluminate 3CaO.Al2O3) does not contribute much to gaining strength and is responsible for the flash setting of cement during hydration. Therefore, gypsum is added to cement to avoid flash setting. Still, celite reacts with gypsum and forms long rod-type crystals of ettringite (3CaO.Al2O3.CaSO4.32H2O), responsible for internal sulphate attack/ cracking in concrete (equation 6) [31-35].
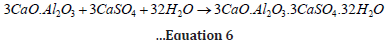
When gypsum is wholly depleted, ettringite, in reaction with C3A and water, converts into monosulphate aluminate hydrate (3C4ASH18), which is 2.5 times smaller than ettringite and forms a membrane around C3A to prevent the further flash setting and reformation of ettringite (equation 7) [31,36]. The 3C4ASH18 can sustain only in sulphate deficient solution and reconverts into ettringite on the absorption of sulphate solution from the atmosphere, which is called external sulphate attack and cause cracking in concrete mass [36].
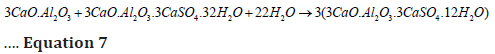
The presence of Ca(OH)2 gives rise to an excess quantity of portlandite in concrete in the form of hexagonal crystals. On the attack of sulphates (MgSO4), an exchange of cations occurs between Ca(OH)2 and sulphates and Mg(OH)2 (brucite) is formed [27,37]. The excess quantity of Ca(OH)2 is generally considered unsuitable for concrete. Still, it produces alkaline solutions and keeps the ‘ph’ value high to avoid acid attacks and corrosion in concrete or steel [27]. Meanwhile, the carbonation of Ca(OH)2 and ettringite starts to form CaCO3 and monocarbonate calcium aluminate hydrates (C3A.CaCO3.11H2O), which adds to the strength, pore refinement, hardening of cement/ lime composite and reduces permeability. Still, strength starts to reduce if it is produced in excess [38,39]. Equations 8 and 9 show the carbonation of reactions [38].

Felite or ferrite (C4AF tetracalcium alumina ferrite 4CaO.Al2O3.Fe2O3) on hydration produces garnets (monosulphate ferric aluminium hydrates) which work as filler material for refined pore structure and does not contribute to strength [31]. Its reaction is completed in two phases. In the first phase, it reacts with gypsum and water to produce ettringite, ferric aluminium hydrates, and portlandite (equation 10). In the second phase, felite reacts with ettringite, water and portlandite to form garnets and ferric aluminium hydrates (equation 11) [31-35]. After complete hydration of Bogue’s compounds, the cement paste contains 50-60% C-S-H gel, 20-25% ettringite, 20-25% portlandite and 5-6% voids/ entrapped air, as shown in Figure 4 [31].

The Synthesis of External/ Internal Sulphate Attacks
The synthesis of cement composition and hydration process in earlier sections explains that the formation of ettringite inside the cement paste by gypsum mixed with clinker causes internal sulphate attack due to its rod-type long crystals that form cracks and propagate a weaker plane throughout the length of damage over an extended period [37]. However, the formation of monocolpate aluminate hydrates prevents more ettringite formation and internal cracking. Later, when cement concrete is exposed to sodium or magnesium sulphate (Na2SO4/ MgSO4) by ingress of sulphate solution through the concrete surface [38] this monocolpate aluminium hydrate reacts with sulphate ions and exchange of cations occurs. It forms ettringite again and exchanges cations and anions to produce CaSO4 (gypsum), brucite Mg(OH)2 and NaOH [37-39]. The attack of sodium sulphate is characterised as an expansive reaction and results in the expansion of the outer surface However, the attack of magnesium sulphate is described as a strength-reducing attack due to internal crack formation by ettringite crystals [39-41]. The cations Mg++/ Na+ and Ca++ exchange, and the anions SO-4 and OH- exchange. The sulphate ion SO-4 from magnesium/ sodium sulphate transfers inwards to form gypsum CaSO4, whereas the OH- ion from Ca(OH)2 exchanges outward to form brucite Mg(OH)2 or NaOH (equation 12,13) [42-46].

The gypsum and brucite produced during external MgSO4 attack initially make a membrane around concrete ingredients to save from sulphate attack [47]. But later, the presence of gypsum initiates the formation of ettringite by reacting with monosulphate aluminium hydrate, which causes peeling off this membrane and propagates inwards, cracking inside the concrete mass (equation 14) [31,36].
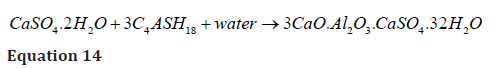
The subsequent diffusion/ exchange of Mg++ cation with Ca++ of calcium silicate hydrates (C-S-H gel) forms magnesium silicate hydrates (M-S-H gel) [42] which do not possess any strength or binding properties and cause a reduction in compressive strength of concrete by reducing intra-ingredients binding (equation 15) [48].
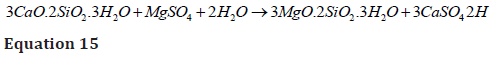
The decrease in intra-ingredients binding and formation/ propagation of cracks by ettringite let the sulphate diffuse deep inside and slowly/ gradually spread this chemical reaction in all dimensions of concrete mass, failing/collapsing the concrete structure [31]. The typical internal sulphate attack is highly influenced by the increased presence of Ca(OH)2 [28], tricalcium aluminates C3A (celite) [36], monosulphate aluminate hydrate (3C4ASH18) [48], which contains a single molar SO-3 [27] and converts readily to ettringite on the formation of gypsum during sulphate attack.
Types, Causes, Sources, Factors and Prevention of Sulphate Attack
Types/ Causes of Sulphate Attacks
The sulphate attack on cement concrete comprises two main internal and external sulphate attack categories. The internal attack is due to the formation of ettringite by the reaction of gypsum used in cement manufacturing to prevent premature/ flash setting due to C3A [31]. However, ettringite so produced is converted into lightweight monosulphate aluminate hydrate after reaction with C3A. This sulphate-deficient molecule is 2.5 times smaller [41]. It gives protection against any damage and further chemical attack till the ingress of sulphate solution in hardened concrete in the form of external sulphate attack from any external source [36]. After that, it swells 2.5 times on gaining sulphate exposure by converting into ettringite crystals resulting in cracking/ expansion [41,49]. The external attack of sulphate transforms monosulphate aluminate hydrate into long ettringite, needle-like crystals, creating cracking inside hardened concrete [50]. In contrast, the formation of micro-ettringite crystals in voids causes expansion in the structures [51]. The magnesium sulphate attack produces gypsum and crumbly fibrous magnesium-silicate-hydrates gel, which does not have any strength and generates the final collapse of the structure [50-51].
Sources of Sulphate Attack
The external source can be soil containing calcium/ magnesium/ sodium/ potassium/ ammonium sulphates [41]. However, solid sulphates cannot impart any harm to hardened concrete [31]. When these minerals are mixed with rain/ drainage water and pass through the soil as groundwater, it dissolves minerals and contains aqueous sulphate. This water attacks the surface and can ingress into the hardened concrete/ mortar structure through osmosis using voids [52]. The bacterial action to decay organic matter in shallow lakes and marshes produces H2S, which is converted into H2SO4 and causes an acid/ sulphate attack [53]. Fertilisers in agricultural soil give rise to ammonium sulphate, which can be transported with surface drainage and into sub-soil groundwater [54]. Sea water also contains sulphate minerals and chlorides and can badly result in sulphate/ chloride attacks [55-57]. The water from cooling towers, sewers, acid rains, and poor chemical waste disposal can result in aqueous sulphates in soil and groundwater [51-55].
Factors and Prevention of Sulphate Attack
The severity of sulphate attack depends upon the quality/ ingredients of cement [31], water/ cement ratio [47], permeability/ voids [41], the concentration/ replenishment of sulphate solution [56], alternate wetting/ drying [57], period of exposure [41], compaction [57], air entrainment [41] etc. The presence of more C3S and less C3A causes vulnerability to sulphate attack. Therefore, reduction in C3S and increased C3A with rich cement ratios are recommended in sulphate resistance cement [60]. The air entrainment in concrete can improve workability and reduce segregation, an excellent preventive measure for sulphate attack [41]. However, lowering C3S can decrease strength as it is responsible for a 60% gain of early strength, and an increase in C3A can result in the flash setting of cement [31]. Similarly, more than 5-6% of air entrainment can cause permeable/ previous structures with lower strength due to the induction of more voids [41]. Therefore, the total percentage of C3S/C2S should not be less than 50%, C3A and C4AF should not be increased by more than 20%, and air entrainment agents should not be used more than 1% to prevent the formation of more than 6% voids [59]. The use of high/super sulphate resistance cement containing more C3A is highly recommended in a rich sulphate environment [60]. A water-cement ratio (w/c) lower than 0.45 is recommended, and no sulphate attacks have been observed on a w/c ratio lower than/ equal to 0.35 [41-47]. Therefore w/c ratio should be kept as 0.35-0.45. Continuous wetting/ drying mechanisms should be avoided. Still, if it is unavoidable, like structures in sea/ rivers, then the coating of bituminous materials [61], chlorinated rubber [43], epoxy or polyurethane [41], use of polyethene or polychloroprene lining are recommended, or the use of super sulphate resistance cement is recommended [60]. The efforts should either reduce or stop the increase in the concentration of sulphate solution or its replenishment by terminating the source of sulphate ingress [62].
Chemical Synthesis of Use of Pozzolans as Partial SCMs in Cement Concrete
Research has focussed on devising composites containing cement, lime [63], and SCMs like industrial/ agricultural pozzolanic materials like pulverised fly ash (PFA) [64], ground granulated blast furnace slag (GGBS) [64], rice husk ash (RHA) [65], palm ash (PA) [65], corn cob ash (CCA) [66], metakaolin (MK) [67], zeolite [68] and silica fume (SF) [67] etc. These materials react with Ca(OH)2 to produce more C-S-H gel and lesser gypsum during sulphate reaction by absorbing portlandite due to the high presence of silicates in their composition (equation16) [28].

However, excess use of pozzolans results in the formation of alkaline silica gel Si(OH)4.CaO. (calcium.silica.hydroxide gel) in the reaction between excess silicate ions SiO- from SiO2 (a significant component of pozzolanic materials) and OH- from portlandite along with the formation of C-S-H gel. The researchers have suggested different optimum quantities of mixing pozzolans and cement replacement materials e.g., up to 70% GGBS [64], 20% MK [67], 40% PFA [64] and 15% SF [67]. Pozzolans with OPC are considered economically viable and environmentally friendly materials [21]. The alkaline silica gel does not contribute to strength but somewhat weakens hardened concrete by creating cracks on swelling (equation 17) Excess SiO2 also reacts with water to convert to silica hydroxide but remains in the pores as an aqueous solution till it further reacts with portlandite (equation 18,19) [69-70]. This alkaline silica gel is formed in four steps SiO2(solid), SiO2(aqueous), SiO2(solution), and swelling SiO2(gel), as shown in equation 20 Therefore, pozzolanic materials contribute to the strength of SCMs to a specific mixing ratio, beyond which they will start to weaken the hardened concrete.


Use of High Sulphate Resistance Cement
The use of high sulphate resistance cement is recommended if construction is undertaken in a rich sulphate environment like bridge/ piers in sea/ rivers/ coastal areas where alternate wetting/ drying is a pronounced phenomenon. The high sulphate resistance cement is type 1 cement as per ASTM 150-C and is manufactured under BS 4027-1980 and BS 12-1996, IS 12330-1988 and EN 197-1/2000 [91]. It contains C3S 40-50%, C2S 20-25%, C3A 5-6% and C4AF 10-15%. SO3 is limited to 3%, MgO is limited to 4%, CaO around 60%, Al2O3 5%, Si2O3 around 20% and lime saturation factor should be 0.6-1% [71]. It produces low heat of hydration and decreases the vulnerability of concrete structures against sulphate attacks in coastal, underwater, underground, sewerage, petrochemical, marshal areas, canal lining etc., prone to sulphate-rich alkaline environments not suitable against chloride attack and not very feasible being a costly option.
Microstructural Analysis and Discussion on Use of Lime, PFA and GGBS
Use of Enhanced Lime-Based Cement Concrete
Sotiriadis et al. (2012) studied using an additional 15% and 35% lime with cement clinker to determine the performance of lime-based concrete against sulphate attack. The lime is the main ingredient in cement manufacturing (62%) and is responsible for supplying CaO and Ca(OH)2 in cement hydration to form C-S-H gel [63]. However, additional mixing of lime in OPC at increased quantities results in the production of more Ca(OH)2 and thaumasite (3CaO.2SiO2.CaCO3.CaSO4.15H2O) during sulphate attack at low temperatures (5oC). Ca(OH)2 creates an alkaline environment and readily converts to crumbling gypsum on cationic exchange with MgSO4, as shown in equations 12 and 13. Thaumasite is produced by the reaction of C-S-H gel with lime under sulphate attack at low temperatures, as shown in equation 21 [63,73].
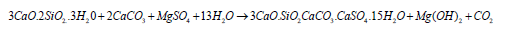
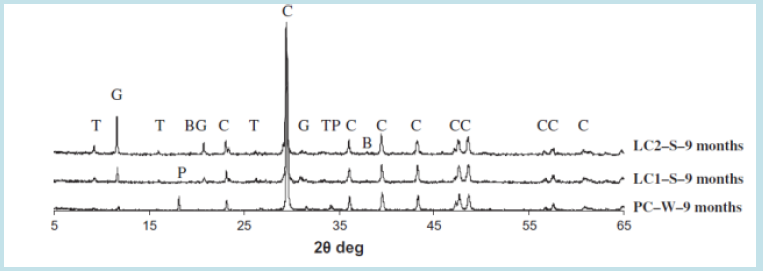
The thaumasite is a complex combination of C-S-H gel with carbonates and sulphate ions bonded with calcium obtained either from additional lime or calcareous sand in concrete at low temperature after the formation of ettringite when presumably aluminates have been consumed by an initial/ internal sulphate attack. Thaumasite has no strength value and rapidly converts concrete into fragile mush and is badly augmented by the formation of M-S-H gel, brucite and gypsum [63-71]. Sotiriadis et al. (2012), conducted a study on mixing additional lime with OPC with a w/binder ratio of 0.52, making 15% mix (LC1) and 35% mix (LC2). A considerable decrease was observed in compressive strength with an increase in lime contents (Figure 5) [63]. They observed a greater degree of surface deterioration in 15% and 35% lime composite over 24 months of immersion in water, 2.5% NaCl+ 2.5% MgSO4 Solution and 5% MgSO4 solutions. The concentrated sulphate solution caused extensive surface damage to cubes of 35% lime, as shown in Figures 6 & 7, as compared to the chloride-sulphate solution because chloride solution dissolves ettringite three times faster and thus lesser C3A is left available to react with sulphates [63]. The microstructural studies using XRD showed that the cement concrete containing 35% lime produced more thaumasite, secondary ettringite, gypsum and brucite in sulphate solution at 5 oC, followed by samples with 15%, and no thaumasite was observed in OPC samples at nine months and 18 months. As a result, concrete deterioration was observed more in samples with more lime contents, and sulphate attack by MgSO4 solution was more severe than NaCl+MgSO4 solution, as shown in (Figures 8 & 9) [63].
Figure 5: Compressive strength of 15% and 35% lime-based concrete cubes immersed in the water, 2.5% NaCl+ 2.5% MgSO4 Solution and 5% MgSO4 solutions for 18 months [63].

Figure 6: 6: Surface deterioration of 15% lime-based concrete cubes immersed in the water, 2.5% NaCl+ 2.5% MgSO4 Solution and 5% MgSO4 solutions for 24 months [63].

Figure 7: Surface deterioration of 35% lime-based concrete cubes immersed in the water, 2.5% NaCl+ 2.5% MgSO4 Solution and 5% MgSO4 solutions for 24 months [63].

Figure 8: XRD study of 15% lime samples in MgSO4 and NaCl+MgSO4 solutions after 18 months (T: thaumasite, c: calcite, BG: brucite gypsum, CC: calcium chloride, B: brucite, G: gypsum) [63].

Figure 9: XRD study of 15% & 35% lime samples in water MgSO4 and NaCl+MgSO4 solutions after nine months [63].
a) Use of Partial Cement Replacements (PCRs)
As discussed earlier, the most prominent/ influential products obtained by hydration of OPC are calcium hydroxide and C-S-H gel [27-30]. The Ca(OH)2 is a highly soluble chemical and dissolves easily in water even after the hardening of concrete resulting in further leaching, porosity and vulnerability to sulphate attack [73]. Therefore, the presence of more Ca(OH)2 pronounces the vulnerability of hardened concrete to external sulphate attack [74]. Research findings have identified that using PCR materials with cement exhibits extended defence against sulphate attack and improves compressive strength [63-67]. The PCRs mixed with OPC react with excess Ca(OH)2. They produce more C-S-H gel, perform as filler material and decrease porosity, prevent ingress of sulphate solution in hardened concrete and stop the propagation of cracks by less formation of ettringite as discussed in earlier section. However, using pozzolans beyond an optimum value produces Si(OH)4, which has swelling characteristics and weakens concrete structure due to expansion [69], as shown in equations 16-20. There are several natural PCRs in use like MK (derived from kaolinite) and zeolite, industrial PCRs like PFA, GGBS, SF and agricultural pozzolans like rice husk ash (RHA), palm ash (PA) and corn cob ash (CCA) which all provide silicates, react with Ca(OH)2 and form additional C-S-H gel for better performance of composites against sulphate attack and strength enhancement [63-83]. Ahmed & Kamau [42]
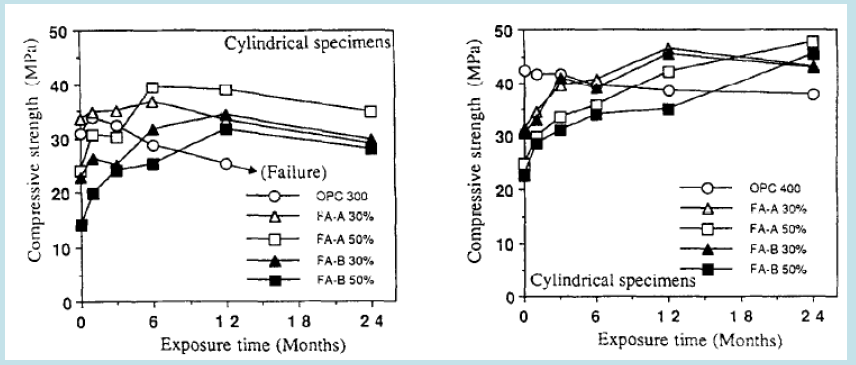
Conducted studies on the use of PFA and GGBS to prepare better-performing pozzolans-based concrete composites, especially in sulphate environments, and they suggested the feasible use of 30% GGBS and 15% PFA for better compressive strengths and sustainability against sodium/ magnesium sulphate solutions [80] in their work on evaluating the performance of PFA-based concrete found it beneficial to use 15-25% PFA for better resistance/ durability of concrete in the aggressive environment against sulphate and chloride penetration [78-80]. studied the mixing of 30% and 50% low and high pulverised fly ash with OPC to produce cubes and prisms of composite mortar and cylinders of composite concrete containing 300 kg/m3 and 400 kg/m3 binders (Figures 10 & 11). The samples were immersed in 5% Na2SO4 solutions for 24 months, and tests were conducted to check compressive strength, expansion and relative dynamic elasticity after 6,12,18 and 24 months. The study found that the samples with only OPC performed the worst and even collapsed after ten months. Whereas samples with 30% fly ash with 400 kg/m3 binder perform well in all testing, followed by 30% high fly ash with 300 kg/m3. The samples with fly ash generally remained stable after six months in sulphate solutions. They did not show much deterioration or expansion after the six months, elucidating that fly ash-based mortar/ concrete composites perform better under sulphate attack due to their tendency to absorb Ca(OH)2, form more C-S-H gel and prevent the formation of ettringite. However, increased use of fly ash resulted in excessive formation of Si(OH)4, which has swelling properties and results in weakness of composites (Figures 12 &13) [76].
Higgis placed prisms of composites of 60% and 70% GGBS with 40% and 30% OPC along with 2% and 3% CaSO4 and 3% CaCO3 in sulphate solutions. Prisms were placed in Na2SO4 solution for 1 year and 3 years. OPC prism performed the worst and showed around 0.1% expansion just after 9 months in Na2SO4. 60% GGBS composite was the second best performer, and 70% GGBS was the first best performer in the Na2SO4 solution Figures 10 & 11 [75] in their studies on the evaluation of the performance of GGBS-based concrete found that 50-60% of GGBS-cement composites performed better in resistance against sulphate and chloride attacks in the aggressive environment [81-83] elucidated that 100%, 50%GGBS+50%PFA and 50% PFA-based geopolymers performed excellent engineering properties especially 100% GGBS-based geopolymer exhibited performance at par OPC. They immersed these pozzolans-based geopolymer/ cement concrete cubes in a 5% H2SO4 solution for 91 days and assessed that a weight loss of 3% was observed in pozzolans-based geopolymers composites compared to an 11% weight loss in OPC concrete supporting a beneficial use of pozzolanic geopolymers/ SCMs over cement concrete [77].
Conclusion
Based on the detailed literature review and study of different experimental works, this paper suggests the following conclusions:
a) The evolution and improvement of construction materials is a continuous process, and more avenues are required to explore to produce environmentally friendly, cost-effective, and robust materials.
b) Cement is the most used construction material and produces around a ton of CO2 per ton of cement during manufacturing, equating to 7-10% emission of global CO2 to put this into perspective, the commercial aviation sector emits only 2.9%.
c) The hydration of cement involves the production of the two most pronounced compounds, i.e., Ca(OH)2 and C-S-H gel. The C-S-H gel is responsible for the strength of concrete and is produced by hydration of C3S, C2S and pozzolans. The portlandite is accountable for an anti-corrosion alkaline environment. Still, it causes a reduction in strength by reacting with reactive metal cations on sulphate attack and converts to gypsum and brucite
d) The internal sulphate attack is caused by gypsum and ettringite, produced during the hydration of cement. The external attack is severe and occurs over an extended period by absorption of sulphate solutions from the atmosphere and soil water, converts monosulphate aluminate hydrates into ettringite and causes expansion and cracks leading to ultimate failure.
e) The concentration of sulphate solution, permeability, period of exposure and cement composition influence the degree of sulphate attack/ deterioration.
f) The water-cement (w/c) ratio of more than 0.45 makes the concrete susceptible to external sulphate attack. In contrast, a w/c ratio of 0.35 and less is the least vulnerable to external sulphate attack even after decades of exposure [41-47].
g) Using GGBS & pozzolans as SCMs is considered beneficial in reducing CO2 emissions and improving strength by producing more C-S-H gel on the chemical reaction of silicates and portlandite [63-67].
h) Pozzolans-based concrete composites are considered better in sulphate resistance due to the depletion of Ca(OH)2 and decreased porosity
i) The use of high sulphate cement and blending of various pozzolanic materials improve the durability of cement concrete composites against sulphate attacks [76-82].
j) The microstructural studies using SEM and XRD support the improvement of pore structures and sulphate resistance with pozzolans [63] as per suggested chemical synthesis in hydration and sulphate attacks explained by equations 4-21 above.
References
- Nadir HM, Ahmed A (2020) Causes and Monitoring of Delays and Cost Overrun in Construction Projects in Pakistan. International Journal of Engineering Invention 8: 20-33.
- Britannica (2020) The Editors of Encyclopaedia. "Concrete". Encyclopaedia Britannica.
- Rehan R, Nehdi M (2005) Carbon dioxide emissions and climate change: policy implications for the cement industry, Environmental Science & Policy 8(2): 105-114.
- Worrell E, Price L, Martin N, Hendriks C, Meida LO, et al. (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26: 303-329.
- (2018) If the cement industry were a country, it would be the third-largest emitter in the world".
- Rajkumar MR (2017) Recent Advances in Materials, Mechanics and Management, in:s 3rd Conf. Mater. Mech. Manag Trivandrum (India) pp. 450.
- Vashisht P, Paliwal MC (2020) Partial Replacement of Cement with Rice Husk Ash in Cement Concrete, International Journal of Engineering Research & Technology 9(12):2278-0181.
- Ahmed A, Hyndman F, Kamau J, Fitriani H (2020) Rice Husk Ash as a Cement Replacement in High Strength Sustainable Concrete, Materials Science Forum, 1007: 90-98.
- NadirHM, Ahmed A (2021) Comparative Evaluation of Potential Impacts of Agricultural and Industrial Waste Pozzolanic Binders on Strengths of Concrete. Journal of Material Sciences & Manufacturing Research.
- Akça KR, Çakir O, Ipek M (2015) Properties of polypropylene fibre reinforced concrete using recycled aggregates. Construct Build Mater 98(15): 620-630.
- Ahmed A, Nadir H, Colin Y, Lee Y (2020) Use of Coconut COIR in Fibre Reinforced Concrete, Soil and Lime 4: 391-399.
- Ty-Mawr Lime Industries Ltd, Sustainable building materials for healthier homes.
- Oates JAH (1998) Projet de. Lime and Limestone - Chemistry and Technology, Production and Uses.
- Peter B (201 3) The lime cycle for high-calcium lime.
- Conserve (2020) STONE TECH (Cleve land) Ltd.t/a Conserve available at https://www.lime-mortars.co.uk/.
- Cement's basic molecular structure finally decoded (MIT, 2009) Archived 21 February 2013 at the Wayback Machine.
- "EPA Overview of Greenhouse Gases". 23 December 2015.
- "The History of Concrete". Dept. of Materials Science and Engineering, University of Illinois, Urbana-Champaign. Archived from the original on 27 November 2012. Retrieved 8 January 2013.
- "EPA Overview of Greenhouse Gases". 23 December 2015.
- "The History of Concrete". Dept. of Materials Science and Engineering, University of Illinois, Urbana-Champaign. Archived from the original on 27 November 2012. Retrieved 8 January 2013.
- Nadir HM, Ahmed A (2021) Comparative Evaluation of Potential Impacts of Agricultural and Industrial Waste Pozzolanic Binders on Strengths of Concrete. Journal of Material Sciences & Manufacturing Research p. 1-8.
- Johannes F (2021) Chemistry of Setting Chapter 10.2.3 in Petroleum Engineer's Guide to Oil Field Chemicals and Fluids (Third Edition).
- Anon (2022) Bogues Compounds | Know 4 Types Of Major Components Of Cement Quickly - Civil Giant.
- com (2020) Chemical Composition Of Cement - Construction How.
- Show D (2020) Cement || Definition, Introduction, Types, Composition and Tests. Mechanical Notes.
- PRODYOGI (2018) Bogue Compounds- Hydration Reactions.
- Anon (2022) What Is Hydration Of Cement? Know Heat Of Hydration & Products Of Cement Hydration Here - Civil Giant. [online] Available at: [Accessed 19 Jun. 2022].
- Nadir HM, Ahmed A (2021) Comparative Evaluation of Potential Impacts of Agricultural and Industrial Waste Pozzolanic Binders on Strengths of Concrete. Journal of Material Sciences & Manufacturing Research p. 1-8.
- Johannes F (2021) Chemistry of Setting Chapter 10.2.3 in Petroleum Engineer's Guide to Oil Field Chemicals and Fluids (Third Edition).
- Shweta G, Devender S (2020) CO2 Sequestration on cement Chapter 6.2.1.1 in Start-Up Creation (Second Edition),
- engr.psu.edu. (n.d.). Hydration.
- Sidney M, Francis Y (1981) Concrete, Prentice-Hall, Inc., Englewood Cliffs NJ pp. 671.
- Steve K & William P (1988) Design and Control of Concrete Mixes, Portland Cement Association, SkokieIll pp. 205.
- Michael M, John Z (1999) Materials for Civil and Construction Engineers, Addison Wesley Longman, Inc.,Jeremy P. Ingham, 5 - Concrete, Editor(s): Jeremy P. Ingham, Geomaterials Under the Microscope, Academic Press, 2013, pp. 75-120.
- Scribd(2018)Hydration of Cement | PDF.
- EldidamonyH El-Sokkari T, Khalil Kh , Heikal, Mohamed, et al. (2012). Hydration Mechanisms Of Calcium Sulphoaluminate C(4)A(3)(S)over-bar, C(4)A(S)over-bar Phase And Active Belite beta-C2S. Ceramics Silikaty 56: 389-395.
- Cefis , Claudia C (2017) Chemo-mechanical modelling of the external sulfate attack in concrete, Cement and Concrete Research p. 57-70,
- Lei M, Peng L, Shi, C and Wang S (2013) Experimental study on the damage mechanism of tunnel structure suffering from sulfate attack. Tunnelling and Underground Space Technology 36: 5-13.
- NevilleA (2004) The confused world of sulfate attack on concrete. Cement and Concrete Research 34(8): 1275-1296.
- Collepardi M (2003) A state-of-the-art review on delayed ettringite attack on concrete. Cement and Concrete Composites 25(4-5): 401-407.
- Anon (2022) Sulphate Attack On Concrete | How To Prevent Sulphate Attack On Concrete? - Civil Giant.
- Ahmed A, Kamau J (2017) Performance of Ternary Class F Pulverised Fuel Ash and Ground Granulated Blast Furnace Slag Concrete in Sulfate Solutions. European Journal of Engineering Research and Science p. 2-8.
- Tixier R, Mobasher B (2003) Modeling of damage in cement-based materials subjected to external sulfate attack, I: Formulation pp. 305-322.
- Idiart AE, Lopez CM, Carol I (2011) Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: a meso-scale model., Cem. Concr. Compos 33: 411-423.
- Marchand I, Older JP (2003) Sulfate Attack on Concrete, CRC Press, USA.
- Santhanam M, Cohen MD, Olek Jb (2022) Mechanism of sulfate attack: a fresh look: part 1: summary of experimental results, Cem. Concr. Res pp. 915-921.
- Stutzman PE, Bullard JW, Feng P (2016) Phase Analysis of Portland Cement by Combined Quantitative X-Ray Powder Diffraction and Scanning Electron Microscopy. Journal of Research of the National Institute of Standards and Technology p. 47.
- Zhang T, Vandeperre LJ, Cheeseman CR (2014) Formation of magnesium silicate hydrate (M-S-H) cement pastes using sodium hexametaphosphate. Cement and Concrete Research 65: 8-14.
- Bai J (2016) Durability of sustainable construction materials. Sustainability of Construction Materials pp. 397-414.
- Panesar DK (2019) Supplementary cementing materials. Developments in the Formulation and Reinforcement of Concrete p. 55-85.
- Yuan Q, Liu Z, Zheng K, Ma C (2021) Inorganic cementing materials. Civil Engineering Materials p. 17-57.
- Echnologyn P (2010) Types of Sulphate Attack in Concrete- Influences and Sources. [online] The Constructor.
- Eštokov A, Harbuláková VO, Luptáková A, Števulová N (2012) Study of the Deterioration of Concrete Influenced by Biogenic Sulphate Attack. Procedia Engineering 42: 1731-1738.
- Beddoe RE, Dorner HW (2005) Modelling acid attack on concrete: Part I. The essential mechanisms. Cement and Concrete Research 35(12): 2333-2339.
- Dezhampanah S, Nikbin ImanM, Charkhtab S, Fakhimi F, Bazkiaei SM, et al. (2020) Environmental performance and durability of concrete incorporating waste tire rubber and steel fiber subjected to acid attack. Journal of Cleaner Production.
- Breysse D (2010) Deterioration processes in reinforced concrete: an overview. Non-Destructive Evaluation of Reinforced Concrete Structures p. 28-56.
- Santhanam M, Otieno M (2016) Deterioration of concrete in the marine environment. Marine Concrete Structures [online] p.137-149.
- Ragab AM, Elgammal MA, Hodhod OA, Ahmed TE (2016) Evaluation of field concrete deterioration under real conditions of seawater attack. Construction and Building Materials 119: 130-144.
- Anon (2021) Chemical Composition of Cement And Functions Of Ingredients Present In Cement - Civil Giant.
- https://www.facebook.com/TheConstructor (2016) Sulphate Attack on Concrete - Process and Control of Sulphate Attack. [online] The Constructor.
- Sarah S, Barbara L, Tilo P, Andreas B, Frank W (2020) Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite, Cement and Concrete Research.
- Sotiriadis K, Nikolopoulou E, Tsivilis S (2012) Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature, Cement and Concrete Composites.
- Ahmed A, Kamau J (2017) Performance of Ternary Class F Pulverised Fuel Ash and Ground Granulated Blast Furnace Slag Concrete in Sulfate Solutions. European Journal of Engineering Research and Science.
- Divya C, Rafat S, Kunal, (2015) Strength permeability and microstructure of self-compacting concrete containing rice husk ash, Biosystems Engineering p. 72-80.
- Kamau J, Ahmed A, Hirst P, Kangwa J (2016) Suitability of Corncob Ash as a supplementary Cementitious Material. International Journal of Materials Science and Engineering 4(4): 215-228.
- Kavitha OR, Shanthi VM, Arulraj G, Prince, Sivakumar VR (2016) Microstructural studies on eco-friendly and durable Self-compacting concrete blended with metakaolin, Applied Clay Science pp. 143-149
- Najimi M, Sobhani J, Ahmadi B, Shekarchi M (2012) An experimental study on durability properties of concrete containing zeolite as a highly reactive natural pozzolan. Construction and Building Materials 35: 1023-1033.
- Ideker C, Thomas JH (2015) Alkali-silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cement and Concrete Research 76: 130-146.
- Powers TC, Steinour HH (1955) An interpretation of some published researches on the alkali-aggregate reaction. Part 1 - The chemical reactions and mechanism of expansion. J Am Concr.Inst 6: 497-516.
- Sulphate Resistant Cement | Standards and Specifications | SRC Grade 42.5.
- McCarthy MJ, Dyer TD (2019) Pozzolanas and Pozzolanic Materials. Lea’s Chemistry of Cement and Concrete. p 363-467.
- Wang J, Cai G. Wu Q (2018) Basic mechanical behaviours and deterioration mechanism of RC beams under chloride-sulphate environment. Construction and Building Materials 160: 450-461.
- Yuan Q, Liu Z, Zheng K Ma C (2021) Inorganic cementing materials. Civil Engineering Materials pp. 17-57.
- Higgins DD (2003) Increased sulfate resistance of ggbs concrete in the presence of carbonate. Cement and Concrete Composites 25(8): 913-919.
- Torii K, Taniguchi K, Kawamura M (1995) Sulfate resistance of high fly ash content concrete. Cement and Concrete Research 25(4): 759-768.
- Jawahar J, Lavanya D, Sashidhar, Chundpalle (2016) Performance of Fly Ash and GGBS Based Geopolymer Concrete in Acid Environment. International Journal of Research and Scientific Innovation 3: 101-104.
- Thomas M (2007) Optimizing use of PFA in cement concrete, published by Portland Cement Association USA in 2007.
- Chi MC, Huang R (2014) Durability Performance of Concrete Containing CFBC Fly Ash and Coal-Fired Fly Ash. Applied Mechanics and Materials pp. 683-287.
- Shannag, MJ, Shaia HA (2003) Sulfate resistance of high-performance concrete. Cement and Concrete Composites 25(3): 363-369.
- Suresh D, Nagaraju K (2015) Ground Granulated Blast Slag (GGBS) in concrete : A review, IOSR Journal of Mechanical and Civil Engineering 4: 76-82
- Shumuye, Eskinder, ZHAO (2018) A Review on Ground Granulated Blast Slag GGBS in Concrete. 5-10.
- Desta E, Jun Z (2018) A Review on Ground Granulated Blast Slag GGBS in Concrete. Eighth International Conference On Advances in Civil and Structural Engineering - CSE 2018.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...