Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Research Article(ISSN: 2641-6921) 
Production Of Silica From Mustard Husk Ash Volume 4 - Issue 3
Mohammad Faris*
- Research Scholar Jamia Millia Islamia New Delhi, India
Received:June 21, 2021; Published: July 8, 2021
*Corresponding author:Mohammad Faris, Research Scholar Jamia Millia Islamia New Delhi, India
DOI: 10.32474/MAMS.2021.04.000188
Abstract
India is the agricultural dominated country where 75% if its population depends upon the agriculture for production of wheat, groundnut, mustard, cotton Tea, Coffee, pulses, soya and Jute. According to the central organization of oil trade (COOTI), 89.5 lakh ton mustard seed was produced during 2020-2021.The Mustard Husk residue easily available in the states which are producing Mustard crop. The mustard seed cellulose after extracting the oil used as the cattle feed supplement which increase the milk extent. The Mustard husk use as the industry fuel for heating the water in boilers for power generation. Agricultural wastes like mustard husk, rice husk, Palm husk and fly husk have the excellent raw material for producing the silica (SiO2). Silica is the Major constituent of Mustard husk ash(MHA) by which it is very become economical for producing silica from MHA. Here we are producing the silica by Citric acid leaching process of mustard husk ash at optimum conditions 800 ˚C and 50 minutes of reaction time & concentration of citric acid above 2%. The produce silica has more than 80-85% purity.
Keywords:Silica; Citric acid; Mustard husk ash; Leaching process; Agriculture
Introduction
Silica is the essential material & important inorganic versatile substance found in crystalline and amorphous forms. The Silica is the amplest material on the earth crust. Todays, most of the silica was produced from quartz or sand by the extraction techniques. Although, production of high purity silica is energy intensified. Additionally, the agriculture wastes have silica as the main constituent after converting these into ash. The scientist is working on producing the silica from agriculture wastes on broad level and which is very essential for reuse of agriculture waste. The leaching process is the best process for production of silica, in the leaching process the substance is removed from the solid by the extraction of liquid. The component is diffuse in the solvent form its natural solid form. The leaching process depends on three parameters like Contact time, solvent selection and the temperature. We can adjust the temperature for ideal solubility and mass transfer. In the leaching process the strong acid also replaced by an organic acid because the organic acid has low dangerous constraints [1-5]. The scientists and researchers from different countries build up environment friendly process to produce silica. The production of mustard husk in India about fifteen million tons obtain form farmers. The burning of Mustard husk causing major health and pollution problem. By the using MHA as raw material for the production of pure Silica full fill the high demand of rubber, cable, footwear, cosmetic and fertilizer industries.
Mustard Crop
The history of the mustard crop very old has been developed as early as a 5000BC.The seed of mustard was found daro-of Harrapan human progress 2300-1750BC. Aryans used Brassica species as pulps and oil. It is very surefire that in a time of more than 3500 years, mustard came to occupy a significant place in the eating regimen of Asian individuals as the wellspring of oils and vegetables. The crop of mustard spread to Europe, Africa, Asia, India and far East [6-10]. In the Asia and Indian subcontinent mustard husk ash used as pesticide in horticulture to protect the crop from insects, mustard oil used as a food item, fuel for boilers generation of electricity, organic manure, animal feed and fuel for bricks industry. the burning of mustard husk leads to high environmental pollution in the world wide. The mustard husk ash has 45.07% of Silica and it is feasible to extract silica from MHA. (Figures 1 & 2)
Experimental Procedure
Leaching process
The MHA was used as the raw material for production of silica, collect from the local agriculture farmhouse. The perpetration of Citric acid solution was done at room temperature by dissolved the powder of citric acid. The mesh size 200 of MHA powder 10 to 25gm put into the 500ml citric acid solution in a beaker. As chelate reaction starts, the metallic impurities depend on the concentration, temperature of solution and the mixing time in solution taking as parameters. The mixing ratio of citric acid powder and water change from 1 to 6%. Now the beaker was placed on the hot plate and temperature changes from 25 to 80 ˚C and time from 25 to 90 mints. The washing process of MHA was done with the distilled water at ambient temperature for removing the citric acid content of ash. The material was dried at 60 ˚C for 60 mints in a furnace after that combusted at 800 ˚C for 30 mints in tube furnace.
Result And Discussion
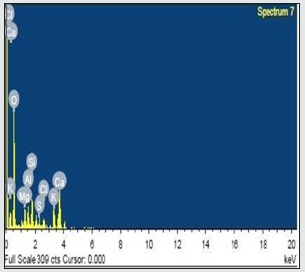
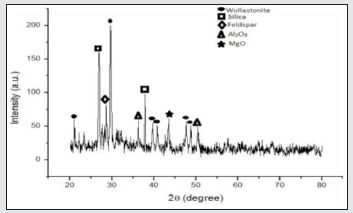
The leaching agents like HCl, H2SO4 and HNO3 used with combination of H2O2 for improving the leaching performance. In the hydrometallurgy these leaching agents are mostly used (Figures 3 & 4). The leaching agents caused environmental pollution, so these are used at optimum conditions. The mustard husk was converted to ash by heating in muffle furnace at 600 ˚C for 60 mints. After that the resulting powder was sieved at 200 mesh size powder was separated. The EDX spectrum of MHA in (Figures 5 & 6) shows presence of silica, alumina and minerals likes wollastonite and feldspar. (Figure 7) XRD). In the XRD the peak was observed at 30 ˚C and shows different phased present in MHA. The presence of also shows peak value. And the low peak value shows the presence of alumina wollastonite etc. Table 1
Effect Of Acid Concentration
The content of SiO2 depends on the concentration of acid. The purity of the silica in the MHA increases upto 92% when the concentration of citric acid is 2% and more. The impurities also reduce as accumulation % of the solution increase. So Al2O3 removed from MHA by leaching more the 2% solution. The MgO content from MHA also decrease up to zero with the concentration above 3%. This is very clear that leaching treatment is very useful for removing the impurities and enhance the purity of Silica in MHA. The leaching agents have the carboxyl groups donating protons (H+) and negative carboxyl groups forms the stable complexes with metal ions. The impurities removed by the reaction of carboxyl groups (-CHOOH) and the metal elements. Table 2
Effect Of Temperature
The impurities and purification of Silica from MHA depends on the temperature also in a such a way that if we increase the temperature up to 80 ˚C the purity of silica increase to 92%. At the temperature 25 ˚C the impurities Al, K and at 80 ˚C MgO removed from MHA at 2% & 3% citric acid concentration respectively .the remaining elements like K ,Ca and Fe discharge into the acid solution during the leaching process. By increasing the temperature, the speed of leaching process increase. The leaching process at the higher temperature effect on citric acid due to their low boiling point temperature. At high temperature the vaporization take places leads to decrease the solubility of reaction and slight effect of corrosion. Table 3
Effect Of Reaction Time
The time also plays a very important role in the leaching process. After the reaction of 25 mints the content of silica increases from 45.60% to 85% [11-15]. If we increase the temperature 25 to 50 mints the content of silica increases up to 92%. However, there is no optimum increase in silica purity more than 50 mints reaction. Table 4
Applications Of Silica
There are various applications in of silica in India. The Silica is broadly divided into rubber and non-rubber grades. Some applications are listed below
1. Raw material for Tires.
2. Act as a shield compound for cables.
3. Raw material for Silicon rubber.
4. Use in toothpaste as cleaning agent.
5. To enhance the mechanical properties of PVC plastics.
6. Constituent for adhesive bonding.
7. Use in footwear soles.
8. Act as dehumidifying agents.
9. Used as silica gel for thermal insulation
10. Use in cosmetics.
Conclusion
The production of mustard crop in India is 17.19 %, soybean, rapeseed –mustard and groundnut are the major oilseed corps in India contributing 84% to 88%. The mustard husk is not a waste material but is also a good source of silica production. The production of silica by this method, waste disposal problem MHA is eliminated. The produce silica has many applications rubber products, paper and composites. Silica is also used as catalyst in the chemical industry and production of silicon also [15-20].
References
- Kalapathy U, Proctor A, Shultz J (2000) A simple method for production of pure silica from rice hull ash. Bioresour Techno 73(3): 257-262.
- Umeda J, Kondoh K (2008) High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J Mater Sci 43: 7084-7090.
- Umeda J, Kondoh K (2008) Environmental Benign Brass Alloys Dispersed with graphite Particles fabricated Via Solid State Sintering Process. Trans JWRI 37(2):
- Umeda J, Imai H, Kondoh K (2009) Polysaccharide Hydrolysis and Metallic Impurities Removal Behavior of Rice Husks in Citric Acid Leaching Treatment. Trans JWRI 38: 13.
- Umeda J, Kondoh K (2010) High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal. Ind Crops Prod 32(3): 539-544.
- Faizul CP, Abdullah C, Fazlul B (2013) Review of Extraction of Silica from Agricultural Wastes Using Acid Leaching Treatment. Adv Mat Res 626: 997.
- Faizul CP, Abdullah C, Fazlul BM (2016) The Utilization of Coconut Fibre into Fired Clay Brick. Key Eng Mater 673: 213-222.
- Habbache N, Alane N, Djerad S, Tifouti (2009) Leaching of copper oxide with different acid solutions. Chem Eng J 152(2-3): 503-508.
- Williams PJ, Biernacki JJ, Rawn CJ, Walker L (2005) J Bai ACI Mater J 102: 330.
- Zainudin NF, Lee KT, Kamarudin AH, Bhatia S, Mohamed AR (2005) Optimization of basic dye removal by oil palm fibre based activated carbon using response surface methodoly. Sep Purif Technol 45: 50.
- Foo KY, Hameed BH (2009) Value-added utilization of oil palm ash: A superior recycling of the industrial agricultural waste. J Hazard Mater 172:
- Fan X, Xing W, Dong H, Zhao J, Wu Y, et al. (2013) Factors Research on the Influence of Leaching Rate of Nickel and Cobalt from Waste Superalloys with Sulfuric Acid. Int J Nonferrous Metal 2(2): 63-67.
- Gharabaghi M, Irannajad M, Noaparats M (2010) A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 103(1-4): 96-107.
- Luke NU, Chinonye II, Terr Aquat (2011) Environ Toxicol 5: 73.
- Ma X, Zhou B, Gao W, Qu Y, Wang L, et al. (2012) A recyclable method for production of pure silica from rice hull ash. Powder Technology 217: 497-501.
- Ugheoke BI, Mamat O, Ari-Wahjoedi B (2013) A direct comparison of processing methods of high purity rice husk silica. Asian Journal of Scientific Research. 6(3): 573-580.
- Vakalova TV, Pogrebenkov VM, Karionova NP (2016) Solid-phase synthesis of wollastonite in natural and technogenic siliceous stock mixtures with varying levels of calcium carbonate component. Ceramics Int 42: 16453-16462.
- Luo Y, Zheng S, Ma S (2018) Novel two-step process for synthesising β-SiC whiskers from coal fly ash and water glass. Ceramics Int 44(9): 10585-10595.
- Tejinder Singh (2000) The Tribun,Industrial Uses of Rice Husk, Chandigarh.
- Perry R, Don Green (1997) “Perry‟s Chemical Engineer‟s Handbook”, 7th (Edn.), McGrow hill Publication, New York.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...