Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Research Article(ISSN: 2641-6921) 
Probing into Dopant Concentration Dependent Luminescence Properties of Transition Metal Mn2+ Activated Ca- α-SiAlON Orange-Red Phosphors Volume 2 - Issue 5
Ni J1,2, Zhou ZZ1, Xu XK1, Liu Q1,2*, Shen FR3,4, and He LH3,5,6**
- 1The State Key Laboratory of High-Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics, Chinese Academy of Sciences, China
- 2Center of Materials Science and Optoelectronics Engineering, Beijing ,China
- 3Institute of Physics, Chinese Academy of Sciences, Beijing , China
- 4School of Physical Sciences, Beijing ,China
- 5Songshan Lake Materials Laboratory, Dongguan, China
- 6Spallation Neutron Source Science Center, Dongguan, China
Received: February 29, 2020; Published: March 11, 2020
*Corresponding author: *Liu Q, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, China
** He LH, Institute of Physics, Chinese Academy of Sciences, Beijing, China
DOI: 10.32474/MAMS.2020.02.000146
Abstract
Transition metal (TM) Mn2+activated Ca-α-SiAlON phosphors with compositions of Ca1-xSi9Al3ON15:xMn2+(x=0.02~0.16) have been designed and synthesized through a high temperature solid-state reaction. In the polyhedron structure of Ca-α-SiAlON host (P31c), Mn2+ions can be accommodated in the lattice without destabalizing the crystal structure, which has been revealed by both XRD and neutron powder diffraction (NPD) characterization. The successful doping of TM Mn2+in host was further confirmed by magnetic susceptibility characterization and the occupation of Mn2+in lattice was analyzed through Rietveld refinement of crystal structure. The slight left-shift of diffraction peaks in XRD patterns and Gaussian fitting sub-bands of emission spectra all proved that the activators Mn2+in Ca-α-SiAlON host not only incorporate into the “separated interstitial sites”, but also modestly substitute for Al3+sites. The phosphors mainly deliver orange-red emission centered at ~600nm under UV excitation, which is ascribed to the typical characteristic 4T1(4G)→6A1(6S) electron transition of divalent Mn2+.The highest photoluminescence (PL) intensity arrives at x=0.12 of Mn2+doping concentration in compositions, then the doping concentration quenching occurs. In addition, through calculating the critical transfer distance, it indicates that the dipole-quadrupole (d-q) interaction is the major mechanism for doping concentration quenching in the phosphors. With the increment of Mn2+content up to x=0.12 (quenching concentration), the decay time τ decreases monotonically from 7.5 to 5.5ms, ascribed to the non-radiative energy migration among Mn2+ ions in the phosphors.
Keywords: Ca-α-SiAlON: Mn2+ phosphor; Magnetic susceptibility; Neutron diffraction; Gaussian fitting; Dipole-quadrupole interaction
Abbreviations: LED: light emitting diodes; CCT: Correlated Color Temperature; TM: transition metal; CRI: Color Rendering Index; NPD :Neutron powder diffraction; TOF: time-of-flight; GPPD: general purpose powder diffractometer; CSNS: China Spallation Neutron Source; GSAS: General Structure Analysis System; MPMS: Magnetic Property Measurement System
Introduction
With the increasing awareness of environmental protection and energy saving, LEDs (light emitting diodes) have gradually became the major source for solid-state lighting. Currently, the commercial white-LED (w-LED) devices sold on the market are using yellow-emitting phosphor (Y3Al5O12:Ce3+ or simply YAG:Ce) with a blue chip (InGaN). The disadvantages of this device, especially for the lamp purpose, are lower Color Rendering Index (CRI, Ra< 75) and higher Correlated Color Temperature (CCT) due to lacking red light [1,2]. To make up for this drawback, researchers have tried to add red phosphors for supplementary, even using red and green phosphor mixture together with a blue chip directly [3,4], or using UV (ultraviolet) chip to excite blue, green, and red phosphor mixture [5]. Hereby, the stable red phosphors with good luminescent properties are highly required in any preparation methods.At present, the red phosphors normally utilize either trivalent rare earth ions such as Eu3+,Pr3+,Sm3+,Tb3+as the luminous centers in sulfides [6], tungstates [7], titanates [8], powellite [9], etc., or divalent Eu2+in nitride M2Si5N8[10] and CaAlSiN3[11]. The chemical stability of the former phosphors is not very good, and the preparation methods of the latter phosphors are a litter difficult. On account of rich oxidation state variation and great optical properties, the transition metal (TM) Mn ions doped oxide, fluoride, sulfide, and nitride phosphors have been extensively investigated [12-31]. Mn ions as activators in varied phosphors may possess different valence states, while the most common is the divalent manganese (Mn2+)or quadrivalent manganese (Mn4+).
Due to the vibrational modes of 2Eg → 4A2g transition for the 3d3 electrons, Mn4+always shows a linear red emission in some oxides [12-15] and fluorides [16-21] with a high correlated color temperature (CCT) and low color rendering index (CRI). As for the Mn2+, it usually shows a broad band emission from green to deep red emission owing to the electron transition of4T1(4G) → 6A1(6S) of Mn2+,in which the lowest excitation level 4T1(4G) decreases with the increase of the crystal field of host, resulting in the red-shift of emission [22,23]. Therefore, the color of emission is strongly dependent on the crystal field strength of the host lattice and the coordination number of Mn2+with neighboring atoms. Generally, Mn2+usually delivers a green emission in weak crystal-field site or with low tetrahedral coordination number [24,25], whereas it shows an orange to deep red emission on a strong crystal-field site or higher octahedral coordination number [26-31]. Namely, the crystal-field and coordination number exhibit a great effect on the emission color of phosphors. Otherwise, the stronger nephlauxetic effect from nitrides and oxynitrides can also enhance the crystal field splitting, which increases the stokes-shift and spectral-shift.Furthermore, the polyhedral oxynitride Ca-a-SiAlON (P31c) is a host material with strong nephlauxetic effect and high coordination number. It possesses an extraordinary chemical and thermal stability, for it consists of stiff three-dimensional tetrahedral [(Si,Al)(N,O)4] networks [32,33]. Accordingly, it is proposed that the smaller Mn2+ion as a promising and abundant non-rare-earth dopant would lead to red emission under excitation when introduced in Ca-α-SiAlON. In the present work, single Mn2+activated Ca-α-SiAlON phosphors were designed and successfully prepared. The dopant’s existence and location, dopant’s concentration effect on emission, and energy transfer mechanism of Mn2+in Ca-α-SiAlON host have been systematically explored, using advanced magnetic susceptibility measurement, neutron diffraction characterization, et al.
Experimental Work
Synthesis of Ca- α-SiAlON: Mn2+ Phosphors
Mn2+single doped Ca-a-SiAlON powders were prepared by a solid-state reaction method. The specific composition is expressed as Ca1-xSi9Al3ON15:xMn2+and Mn2+doping concentrations range from 0.02 to 0.16 (x=0.02, 0.04, 0.06, 0.08, 0.10, 0.12, 0.14, 0.16). The starting materials used in preparation of these samples were α-Si3N4 (SN-E10, Ube Industries, Japan), AlN (Grade A, Starck Industries, German), CaCO3(Sinopharm Chemical Reagent Co., Ltd, china) and MnCO3 (Sinopharm Chemical Reagent Co., Ltd, china). The pre-weighed powders were mixed up by ball-milled method in ethanol for 12h. Then the mixture powders were dried at 80°Cfor6h. After entirely ground, the appropriate mixture powders were transferred into BN crucibles and calcined at 1600°C for 4h under N2atmosphere in a graphite furnace at a heating rate of 5°C/min. The calcination temperature of 1600°C/4h is an optimal parameter based on our previous work. Finally, the cooled powders were ground twice for further measurements and characterizations.
Characterization and Measurements
The crystal phase of as-prepared samples were identified by X-ray diffractometer (D8 ADVANCE, Bruker, Germany) with CuKαradiation (λ=0.1541 nm), in a 2q range of 20o to 80o. Neutron powder diffraction (NPD) was performed at time-of-flight (TOF) general purpose powder diffractometer (GPPD) at China Spallation Neutron Source (CSNS). Neutron diffraction patterns were collected at room temperature with wavelength band from 0.1 to 4.9Å. The crystal structure and occupation of Mn ions in the host were refined by the Rietveld method using the General Structure Analysis System (GSAS) suite of programs. The magnetic susceptibility was tested by Magnetic Property Measurement System (MPMS, SQUID, Quantum Design, USA) to confirm the successful doping of magnetic TM Mn2+ions in host. The photoluminescence measurements (excitation and emission spectra, PLE and PL) of phosphors Ca1-xSi9Al3ON15:xMn2+with varied Mn2+concentration (x=0.02q0.16) were performed at room temperature to determine the doping concentration dependent photoluminescence properties, on a F-4600 spectrometer (Hitachi, Japan) with a 150 W xenon lamp as excitation. The decay curves of Mn2+lifetime values were recorded on a lifetime and steady state spectrometer (FLS 980, Edinburgh Instruments, UK), with a lifetime measurement range of 1qs q10s (μF2).
Results and Discussion
Crystal Structure of Ca- α -SiAlON: Mn2+ Phosphors
It has been found that all the prepared phosphors Ca1-xSi9Al3ON15:xMn2+are isostructural and crystallized in its hexagonal host Ca-α-SiAlON (P31c), as shown in Figure 1, with a standard reference XRD pattern of Ca-α-SiAlON at the bottom of the figure (JCPDS card No. 33-0261). However, among the different Mn2+concentration, the prepared compounds show a high-purity phase with a lower Mn2+doping concentration x=0.02 and 0.04. The trace of impurity phase is found when the doping concentration increases above 0.04. The impurity phase is identified as AlN and marked with “®” in(Figure1) (AlN: JCPDS card No.25-1133), which is derived from the residual incompletely reacted AlN raw material. This phenomenon reveals a quite low solid solubility of Mn2+ions in Ca-α-SiAlON host and the solubility plays an important role in the solid-state reaction to synthesize highly pure Ca-α-SiAlON:Mn2+phase at 1600°C. Theoretically, there exist two specially “separated interstitial sites” per unit cell in the α-SiAlON crystalline structure, one is a rectangle site with 0.10x 0.24 nm in two-dimensional scale and another is a circular site with a 0.15nm diameter [34]. Relative to the Mn2+ion radius (0.067nm in ionic radius), the interstitial site is large enough to be occupied by Mn2+ion.
Figure 1: (a) XRD patterns of Ca-α-sialon: xMn2+ (x=0.02-0.16) phosphors synthesized at 1600℃, (b) the magnified regions of XRD patterns in a 2θ range of 33o ∽ 36o.
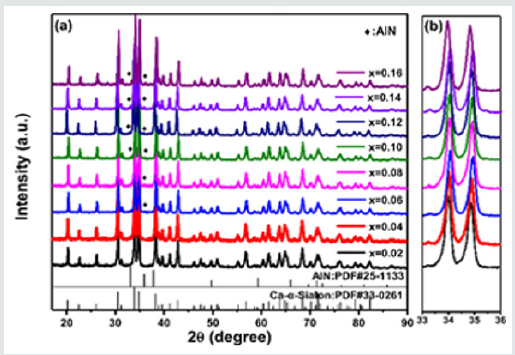
Presuming that all the introduced small Mn2+ions prefer to substitute Ca2+sites (normally occupied at the interstitial sites) or incorporate into the unoccupied separated interstitial sites in Ca-α-SiAlON host, in this case, the XRD diffraction spectra would not be changed and lattice parameters could not be influenced. However, under accurate examination, it has been found that the XRD diffraction peaks in the selected 2θ region from 33o to 36ogradually shift to lower 2θ angles with increasing of Mn2+concentration up to x=0.16 (seen in Figure1b), implying a slight lattice expanding caused by a portion of Mn2+ions substituting for smaller Al3+(0.0535nm in ionic radius) or perhaps Si4+ (0.0400nm in ionic radius). From another angle, in Ca-α-SiAlON host, the bond length of Al-N, Al-O, Si-N and Si-O was estimated to be ~1.87 Å, ~1.75 Å, ~1.74 Å, and ~1.62 Å, respectively [35-37], while, the bond length of Mn-N and Mn-O was statistic to be ~1.86Å and ~1.79Å in some nitrides and oxides [38-41]. Therefore, Mn2+ions have more possibilities to replace Al3+sites (MnAl) rather than Si4+sites. This result further demonstrates that the introduction of more Mn2+ions could result in a difficult solid-solution and then hamper the reaction of AlN with other starting powders, which produced the AlN impurities ultimately.Besides, in order to further confirm the incorporation and location of Mn2+in its host, neutron powder diffraction (NPD) studies were performed for the compound Ca-α-SiAlON:0.12Mn2+.Among all the prepared compounds, we selected the compound of Ca-α-SiAlON:0.12Mn2+, because it shows the highest emission intensity under UV excitation. The NPD patterns were collected at room temperature by time-of-flight (TOF) general purpose powder diffractometer (GPPD).
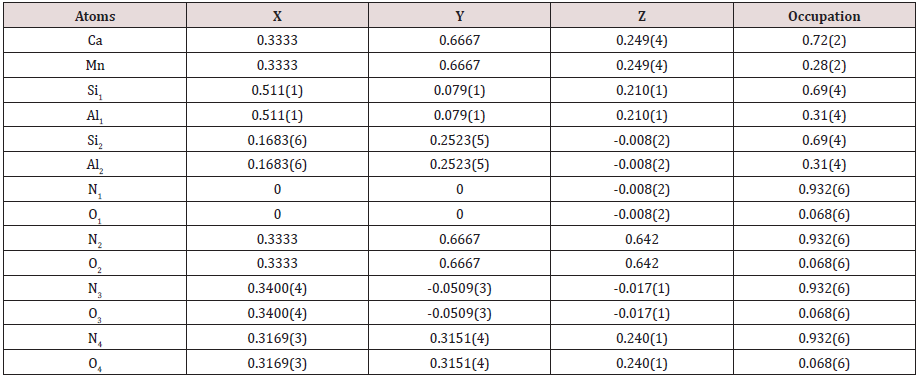
In (Figure2), it shows the NPD pattern of Ca-α-SiAlON:0.12Mn2+, together with Bragg position, the calculated pattern, and the difference between experimental and calculated patterns. The atomic positions and occupations in the compound Ca-α-SiAlON:0.12Mn2+from detailed refinement results of the NPD pattern are listed in Table1, from which it can been seen that the doped emitting centers of Mn2+are almost occupied at Ca2+sitesIn addition, to confirm the successful dilute doping of Mn2+in Ca-α-SiAlON matrix, the magnetic susceptibility measurement, which is sensitive to magnetic TM Mn2+ addition, was carried out and the results of Moment-Magnetic Field (M-H) curves are indicated in(Figure3), for three comparative compounds: un-doped Ca-α-SiAlON, low-doping Ca-α-SiAlON:0.02Mn2+,and heavy-doping Ca-α-SiAlON:0.12Mn2+.Normally, the Ca-α-SiAlON host as a semiconductor is a type of anti-magnetic material, however, it gradually turns into a ferromagnetic material as the Mn2+ doping concentration increases, as shown in Figure 3. According to the magnetic susceptibility results measured at 2K and 300K for the three comparative compounds, the magnetic susceptibility change also proves that Mn2+ions have been incorporated into the lattice of host.
Figure 2: Neutron powder diffraction pattern of Ca-α-sialon:0.12Mn2+, together with Bragg position (black circle), the calculated pattern (red line), and the difference (purple line) between experimental and calculated patterns, and peak position (black bar), collected at room temperature by GPPD.
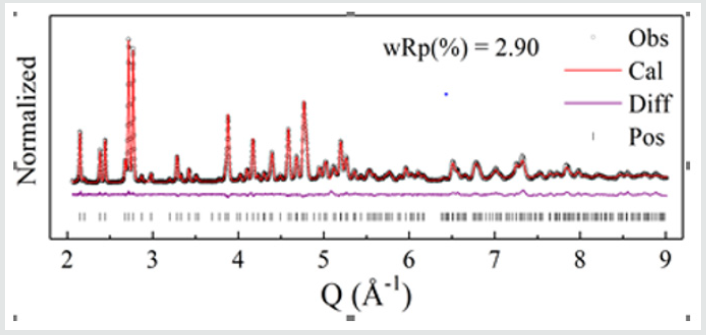
Figure 3: Magnetic susceptibility of M-H curves on, (a) un-doped Ca-α-sialon host; (b) Ca-α-sialon:0.02Mn2+ phosphor, and (c) Ca-α-sialon:0.12Mn2+ phosphor.
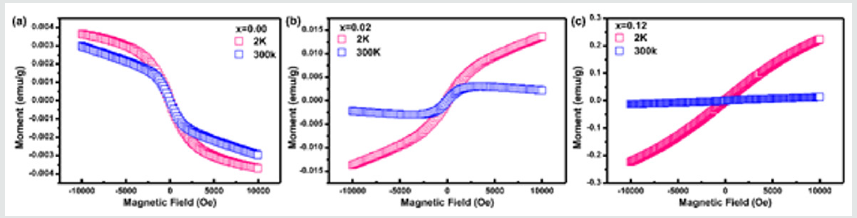
Table 1: Atomic positions and occupations of Ca-α-sialon: 0.12Mn2+, with lattice constants a=b=7.8565(2)Å, c=5.7166(1)Å.
Luminescence Properties of Ca-α-SiAlON: Mn2+ Phosphor
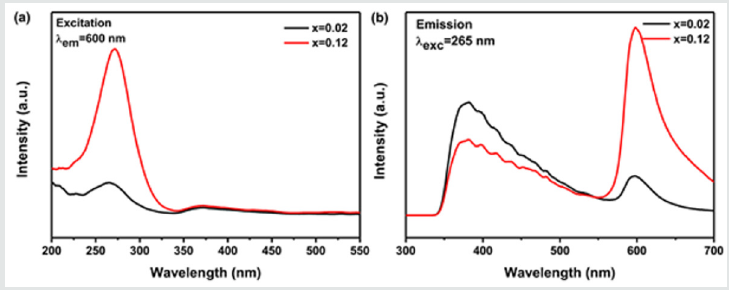
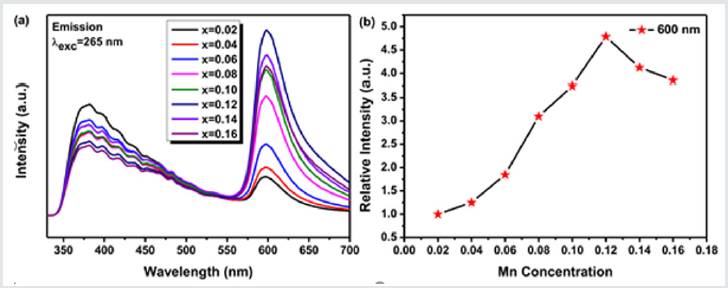
The representative photoluminescence excitation and emission spectra of Ca-α-SiAlON:0.02Mn2+and Ca-α-SiAlON:0.12Mn2+phosphors are illustrated in (Figure 4)4a &4b. The excitation spectra monitored at 600nm comprise one dominant band centered at 265nm and other weak bands between 350nm to 450nm. The 265nm band is related to the band gap absorption [42] and the weak bands are ascribed to the transitions of Mn2+from the ground state 6A1(6S) to excited states 4T2(4D), [4A1(4G), 4E(4G)] and 4T1(4G) levels, respectively [25,43-45]. Because d-d electron transition of Mn2+is parity spin-forbidden, these excitation bands between 350nm to 450nm are very weak. For all the prepared compounds, two wider and stronger emission peaks are observed in PL spectra under UV light excitation of 265nm. One stronger dominant orange-red emission with a maximum at ~600nm is from the typical emission of Mn2+,corresponding to the energy transition 4T1(4G) →6A1(6S) of Mn2+in the highly coordinated nitride and oxide host lattice [25,45-48]. Another emission band ranging from 350nm to 550nm shows a wider UV-green emission possibly assigned to the oxygen-related defects (mainly oxygen vacancy) in the host lattice. The presumed oxygen defects have elucidated and proved by the Electron Paramagnetic Resonance (general purpose powder diffractometer) spectra examination in another separated work [42]. The oxygen-related defects are possibly developed in the high temperature synthesis process of phosphors in a reductive N2environment.Otherwise, the strong orange-red emission band peaked at ~600nm is apparently asymmetry and could be divided into several emissions.
Figure 4: Excitation (λem=600nm); and emission (λexc=265nm); spectra of Ca-α-sialon:0.02Mn2+ and Ca-α-sialon:0.12Mn2+ phosphor samples.
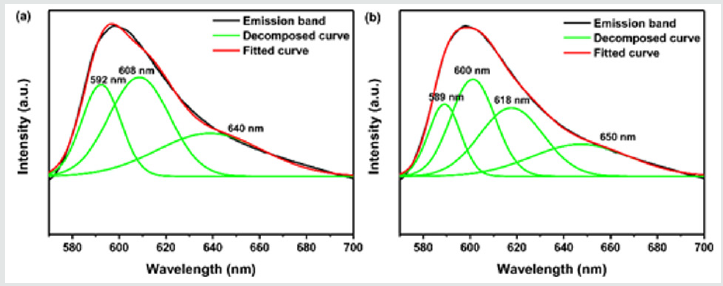
If only the interstitial sites in Ca-α-SiAlON host are substituted by Mn2+ions, perhaps it is expected to just show three narrow symmetrical emission bands in the luminescence spectra (depicted in Figure5a), since Mn2+ions occupied at lattice interstitial sites were proposed to be coordinated by seven (O,N) anions in three different M-(O, N) distances, named as Mn(1), Mn(2), and Mn(3), based on the crystal structure refinement analysis [49]. While, contrasting to Figure 5b, the orange-red emission should be better separated into four sub-bands by Gaussian fitting, which are centered averagely at 589nm, 600nm, 618nm and 650nm, respectively, demonstrating four different emission sites in the representative Ca-α-SiAlON:0.12Mn2+phosphor. According to the above-mentioned, the emission bands from lower energy (600 nm or 16666cm-1, 618nm or 16181cm-1, and 650nm or 15384cm-1) should originate from the Mn2+locating at interstitial sites, they are Mn(1), Mn(2), and Mn(3) with seven (O,N) anions coordination [49] (Figure 5). Another emission band from higher energy (589nm or 16978cm-1) might be assigned to the Mn2+locating at Al3+ sites (MnAl) with four-fold (N.O) coordination [35-37]. The Gaussian fitting results of the asymmetry emission band in the Ca-α-SiAlON:0.12Mn2+compound is quite similar to that in CaAlSiN3:Mn2+nitride phosphors [28]. In CaAlSiN3:Mn2+,there is only one crystallographic site for Ca2+coordinated with five N and one site for Al3+coordinated with four N. Thus, when heavily-doping,Mn2+ions can occupy not only at pentahedrally coordinated Ca site (MnCa), but also at tetrahedrally coordinated Al site (MnAl) to some extent, then the asymmetry emission band is deconvoluted into two Gaussian sub bands centered at about 548nm and 627nm, respectively.
Figure 5: Emission (λex=265nm) spectra of Ca-α-sialon:0.12Mn2+ phosphor sample with Gaussian fitting components: (a) separated into three sub-bands, (b) separated into four sub-bands.
Concentration Quenching and Decay Behavior of Ca-α- SiAlON: Mn2+ Phosphors
The dependence of emission intensity (peaked at ~600 nm) on Mn2+ion concentration is given in (Figure6). The emission intensity is maximized at the Mn2+concentration around 12mol% and it is nearly five times of that for Ca-α-SiAlON:0.02Mn2+,then the emission intensity diminishes with further increase of Mn2+content, indicating a clear concentration quenching of emission above 12mol% of Mn2+content. The concentration quenching phenomenon may be caused by the re-absorption of Mn2+ions at a higher doping level and non-radiative energy transfer between Mn2+and Ca-α-SiAlON host. Hence, the doping concentration dependent emission is very important for evaluating phosphors’ properties. To deeply investigate the concentration quenching phenomena of our Ca-α-SiAlON:xMn2+phosphors, the critical transfer distance (Rc) related to the Mn2+concentration quenching can be estimated from the equation as follows, on account of the mechanism of energy transfer in phosphors pointed by Blasse-formula [50, 51]:
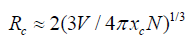 (1)
(1)
Figure 6: (a)Emission (λexcel=26 nm) spectra of Ca-α-sialon:xMn2+ phosphors with various x from 0.02 to 0.16, (b) The curve of PL intensity of Ca-α-sialon: xMn2+ around ~600nm emission as a function of Mn2+
Using this equation, V = 0.308nm3 is the volume of the unit cell of Ca-α-SiAlON, N=2 is the number of dopant in the unit cell of Ca-α-SiAlON, and xc = 0.12 is the critical quenching concentration of Mn2+.The critical transfer distance (Rc) is calculated to be ~13.48Å, which is much longer than the typical critical distance (normally~5Å for most phosphors [52])responsible for forbidden transitions of Mn2+via exchange interaction. Therefore, in Ca-α-SiAlON:xMn2+,it hardly plays any role on the energy transfer among Mn2+ions through exchange interaction. As a result, it is inferred that the concentration quenching does not take place via exchange interaction, while it is mainly governed by electric multipolar interaction, such as the situation in Eu2+doped Ca-α-SiAlON[53-54]. Except the Blasse-formula, the critical distance Rc could be also obtained from Dexter’s formula and given as follows [52,55]:
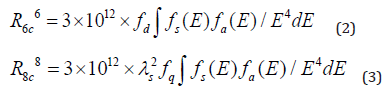
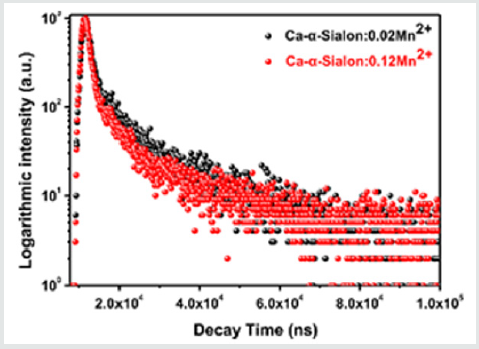
Here, R6c represents the dipole-dipole energy transfer distance, R8c represents the dipole-quadrupole energy transfer distance, ƒd = 10-7 and ƒq = 10-10are the oscillator strength for dipole-dipole and dipole-quadrupole electrical absorption transitions [56]. Then, the spectra overlap ∫ƒs(E)ƒa(E)/E4dE is estimated to be 0.0586eV-5, which is extracted experimentally from the normalized excitation and emission spectra datum of our Ca-α-SiAlON:0.12Mn2+compound, λs = 6000Å is the wavelength of maxima emission peak. Moreover, Eq.(2) indicates the dipole-dipole energy transfer mechanism between Mn2+and Ca-α-SiAlON host, while Eq. (3) indicates the dipole-quadrupole energy transfer mechanism. According to the above formula (2), R6c is calculated to be 5.1Å, which largely deviates from the result obtained from Blass-formula calculation result. Since the 3d electron transitions of Mn2+is spin-forbidden, the possibility of a dipole-dipole interaction is very small in Mn2+single doped host [57]. Whereas, R8c is calculated to be 12.60Å, which is approximate to the previous datum of 13.48Å using Blass-formula calculation. Therefore, it is confirmed that the dipole-quadrupole (d-q) interaction is themajor mechanism for concentration quenching in Ca-α-SiAlON:xMn2+phosphors.To declare the luminescence lifetime of the produced Ca-α-SiAlON:xMn2+phosphors, the room temperature lifetime curves of Mn2+in Ca-α-SiAlON host (x=0.02 and 0.12) monitored at 600nm with 265nm excitation were measured and are displayed in (Figure 7). The decay curves could be approximately assessed using the empirical equation:
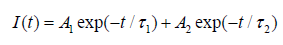 (4)
(4)
Figure 7: Decay curves of Ca-α-sialon:0.02Mn2+ and Ca-α-sialon:0.12Mn2+ phosphors under 265nm excitation and monitored at 600nm emission.
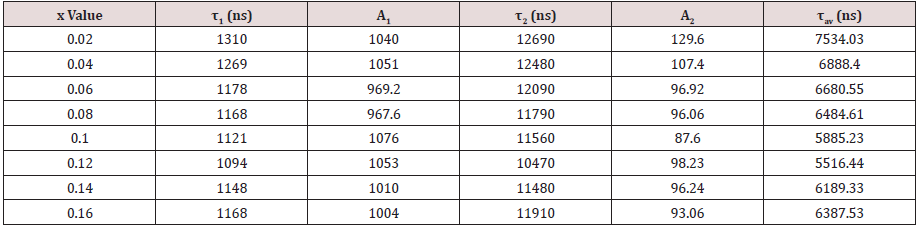
In this equation, I mean luminescence intensity; A1 and A2 are constants; t is testing time; τ1 and τ2 are the shorter and longer decay time calculated by Eq. (4). According to the above equation and our conducted computer fitting, τ1 and τ2 for the all prepared samples are listed in Table2. The average lifetime constant (τ) can be estimated using the equation (5):
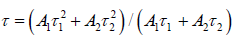 (5)
(5)
On the basis of Eq. (5), with the increment of Mn2+content up to x=0.12 (concentration quenching), the decay time τ decreases monotonically from 7.5ms to 5.5msshown in Table2, ascribed to the non-radiative energy migration among Mn2+ions. Otherwise, the decay curves fit well with the double-exponential decay mode, further confirming that it has more than one of luminescence center sites in Mn2+doped Ca-a-SiAlON phosphors.
Table 2: The calculation results of short decay time, long decay time, and average decay time for Ca-α-sialon: xMn2+ (x=0.02-0.16) phosphors under 265nm excitation and monitored at 600nm emission.
Conclusion
Transition metal Mn2+ single activated Ca-α-SiAlON orange-red phosphors have been successfully synthesized by solid-state reaction method. Research results on dopant concentration dependent luminescence properties of the phosphors can be summarized as follows: (1) All the prepared phosphors Ca1-xSi9Al3ON15:xMn2+are isostructural and crystallized in its hexagonal host Ca-α-SiAlON (P31c), and emission centers Mn2+ have been incorporated into the lattice of host with Mn2+mainly occupied at Ca2+sites, conformed by using XRD analysis, neutron powder diffraction measurement, and magnetic susceptibility characterization.(2) The prepared phosphors exhibit a dominant asymmetry orange-red emission centered at ~600nm under 265nm (UV) excitation and the asymmetry emission band is deconvoluted into four Gaussian fitted sub-bands centered at 589nm, 600nm, 618nm and 650nm, respectively, indicating four different Mn2+sites in Ca-α-SiAlON host. (3) It arrives the highest PL emission intensity at x=0.12 of Mn2+doping concentration in Ca1-xSi9Al3ON15:xMn2+ compositions, that means the luminescence quenching concentration critical value x=0.12 in the series of Ca1-xSi9Al3ON15:xMn2+compounds. (4) A litter far distance (R8c = 12.60Å) energy transfer model via the dipole-quadrupole interaction has been deduced and considered to be the major mechanism for doping concentration quenching in Ca-α-SiAlON:xMn2+phosphors. (5) With the increment of Mn2+content up to x=0.12 (concentration quenching), the decay time τ decreases monotonically from 7.5ms to 5.5ms, ascribed to the non-radiative energy migration among Mn2+ions in the phosphors.
Acknowledgement
Many thanks for financial supports from the National Key Research and Development Program of China (Grant No. 2017YFB0703203) and the Research Program of Shanghai Sciences and Technology Commission Foundation (Grant No. 18511110400).
References
- HolonyakN, BevacquaSF (1962) Coherent (visible) Light Emission fromGa (As1−xPx) Junctions. ApplPhys Lett1(4): 82-83.
- Lin, CC, ZhengYS, ChenHY, RuanCH (2010)Improving Optical Properties of White LED Fabricated by a Blue LED Chip with Yellow/Red Phosphors. JElectrochemSoc157(9): H900-H903.
- Wu H, Zhang XM, Guo CF, Xu J (2005) Three-band white light from InGaN-based blue LED chip precoated with Green/red phosphors. IEEE Photonics TechLett17(6): 1160-1162.
- HoppeHA (2009) Recent developments in the field of inorganic phosphors. AngewChemIntEdEngl48(20): 3572-3582.
- SheuJK, Chang SJ, KuoCH, SuYK (2003) White-light emission from near UV InGaN-GaN LED chip precoated with blue/green/red phosphors. IEEE Photonics TechLett15(1): 18-20.
- Jia D, Hunter DN (2006) Long persistent light emitting diode. J Appl Phys100(11): 113-125.
- Wen D, Feng J, Li J, Shi J (2015) K2Ln(PO4)(WO4):Tb3+,Eu3+ (Ln = Y, Gd and Lu) phosphors: highly efficient pure red and tuneable emission for white light-emitting diodes. JMater ChemC 3(9): 2107-2114.
- Zhang JC, Wang XS, Yao X (2010) Enhancement of luminescence and afterglow in CaTiO3:Pr3+ by Zr substitution for Ti. J Alloys Compd498(2): 152-156.
- Thomas M, Prabhakar Rao P, Deepa M, Chandran MR, et al. (2009) Novel powellite-based red-emitting phosphors: CaLa1−xNbMoO8:xEu3+ for white light emitting diodes. J Solid State Chem182(1): 203-207.
- Schlieper T,Milius W,Schnick W (1995)Nitrido-silicateII[1]Hochtemperatur-Synthesen und Kristallstrukturen von Sr2Si5N8 und Ba2Si5N8. Zeitschriftfüranorganische und allgemeineChemie 621(8): 1380-1384.
- Piao X, Machida K, HorikawaT, Hanzawa H (2007) Preparation of CaAlSiN3:Eu2+ Phosphors by the Self-Propagating High-Temperature Synthesis and Their Luminescent Properties. Chem Mater19(18): 4592-4599.
- Wang B, Lin H, Xu J, Chen H (2014) CaMg2Al16O27:Mn4+-based red phosphor: a potential color converter for high-powered warm W-LED. ACS ApplMater Inter 6(24): 22905-22913.
- Kim SJ, Jang HS, UnithrattilS, Kim YH (2016) Structural and luminescent properties of red-emitting SrGe4O9:Mn4+ phosphors for white light-emitting diodes with high color rendering index. J Lumin172: 99-104.
- Peng M, Yin X, Tanner PA, Liang C (2013) Orderly-Layered Tetravalent Manganese-Doped Strontium Aluminate Sr4Al14O25:Mn4+: An Efficient Red Phosphor for Warm White Light Emitting Diodes. JAmCeram Soc96(9): 2870-2876.
- Lu W, Lv W, Zhao Q, Jiao M (2014) A novel efficient Mn4+ activated Ca14Al10Zn6O35 phosphor: application in red-emitting and white LEDs. InorgChem53(22): 11985-11990.
- Kim M, Park WB, Bang B, Kim CH (2015) Radiative and non-radiative decay rate of K2SiF6:Mn4+ J MaterChemC 3(21): 5484-5489.
- Arai Y and Adachi S (2011) Optical properties of Mn4+-activated Na2SnF6 and Cs2SnF6 red phosphors. JLumin131(12): 2652-2660.
- Sekiguchi D, Nara J, Adachi S (2013) Photoluminescence and Raman scattering spectroscopies of BaSiF6:Mn4+ red phosphor. JApplPhys113(18): 183-516.
- Verstraete R, Sijbom HF, Joos JJ, Korthout K(2018) Red Mn4+-Doped Fluoride Phosphors: Why Purity Matters. ACS Appl Mater Inter 10(22): 18845-18856.
- Zhu YW,Shuo Y,Huang L,Liu Y (2019) Optimizing and adjusting the photoluminescence of Mn4+-doped fluoride phosphors via forming composite particles. Dalton Trans48(2): 711-717.
- Fang MH,Yang TH,Lesniewski T, Lee C (2019) Hydrogen-Containing Na3HTi1–xMnxF8Narrow-Band Phosphor for Light-Emitting Diodes. ACS Energy Lett4(2): 527-533.
- Palumbo DT,BrownJJ(1970)Electronic States of Mn2+-Activated Phosphors. JElectrochemSoc117(9): 1184.
- Kim JS, Kim JS, Kim TW, Kim SM (2005) Correlation between the crystalline environment and optical property of Mn2+ ions in ZnGa2O4:Mn2+ phosphor. ApplPhys Lett86(9): 91912-91913.
- Lei B, Li B, Wang X, Li W (2006) Green emitting long lasting phosphorescence (LLP) properties of Mg2SnO4:Mn2+ JLumin118(2): 173-178.
- Shang M, Li G, Yang D, Kang, X (2011) (Zn,Mg)2GeO4:Mn2+submicrorods as promising green phosphors for field emission displays: hydrothermal synthesis and luminescence properties. DaltonTrans40(37): 9379-9387.
- Shi L, Huang Y, Seo HJ (2010) Emission red shift and unusual band narrowing of Mn2+ in NaCaPO4 JPhys ChemA 114(26): 6927-6934.
- Polikarpov E, Catalini D, Padmaperuma A, Das P (2015) A high efficiency rare earth-free orange emitting phosphor. OptMater46: 614-618.
- Zhang ZJ, Delsing ACA, Notten PHL, Zhao JT(2013) Photoluminescence Properties of Red-Emitting Mn2+-Activated CaAlSiN3Phosphor for White-LEDs. Ecs J Solid State Sc 2(4): R70-R75.
- Lin YF, Chang YH, Chang YS, Tsai BS (2006) Photoluminescent properties of Ba2ZnS3:Mn phosphors. JAlloys Compd421(1-2): 268-272.
- Duan CJ, Otten WM, Delsing ACA, Hintzen HT (2008) Preparation and photoluminescence properties of Mn2+-activated M2Si5N8(M=Ca, Sr, Ba) phosphors. J Solid State Chem181(4): 751-757.
- Collins BT(1993)Synthesis and Cathodoluminescence of Orange-Yellow- toRed-Emitting Ca(1-x)MgxS:Mn Phosphors. JElectrochem Soc140(6): 1752.
- Xie RJ, Hirosaki N, Li Y, Takeda T (2010) Rare-Earth Activated Nitride Phosphors: Synthesis, Luminescence and Applications. Mater 3(6): 3777-3793.
- Liu L, Xie RJ, Hirosaki N, Takeda T (2009) Temperature Dependent Luminescence of Yellow-Emitting α-Sialon:Eu2+ Oxynitride Phosphors for White Light-Emitting Diodes. JAmCeramSoc92(11): 2668-2673.
- Grün R (1979) The crystal structure of β-Si3N4: structural and stability considerations between α- and β-Si3N4. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 35 (4): 800-804.
- Xie RJ, Hirosaki N, Mitomo M, Yamamoto Y (2004) Optical properties of Eu2+ in α-SiAlON. JPhys Chem B 108(32): 12027-12031.
- Jack KH (1976) Sialons and related nitrogen ceramics. JMater Sci11(6): 1135-1158.
- Hampshire S, Park HK, Thompson DP, Jack KH (1978) α'-SiAlON ceramic. Nature 274(5674): 880-882.
- Thorp HH (1992) Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. LnorgChem31(9): 1585-1588.
- Liu W, Thorp HH (1993) Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes2:Refined distances and other enzymes. lnorg Chem 32 (19): 4102-4105.
- Palenik GJ (1997) Bond Valence Sums in Coordination Chemistry Using Oxidation State Independent R0 Values A Simple Method for Calculating the Oxidation State of Manganese in Complexes Containing Only Mn−O Bonds. LnorgChem 36(21): 4888-4890.
- Brown ID, Wu KK (1976) Empirical parameters for calculating cation–oxygen bond valences. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 32(7): 1957-1959.
- Ni J, Liu Q, Wan JQ, Liu GH (2018) Novel Luminescent Properties and Thermal Stability of Non-Rare-Earth Ca-α-sialon:Mn2+ J Lumin202: 514-522.
- Wang XJ, Xie RJ, Dierre B, Takeda T (2014) A novel and high brightness AlN:Mn2+ red phosphor for field emission displays. Dalton Trans 43(16): 6120-6127.
- Duan CJ, Delsing ACA, Hintzen HT (2009) Photoluminescence Properties of Novel Red-Emitting Mn2+-Activated MZnOS (M = Ca, Ba) Phosphors. Chem Mater 21(6): 1010-1016.
- Shi Z, Zou Y, Jing R, Zhang K (2015) Red-emitting AlN:Mn2+ phosphors prepared by combustion synthesis. FuncMaterLett8(2): 1550025.
- Duan CJ, Delsing ACA, Hintzen HT (2009) Red emission from Mn2+ on a tetrahedral site in MgSiN2. JLumin 129(6): 645-649.
- An JS, Noh JH, Cho IS, Roh HS (2010) Tailoring the Morphology and Structure of Nanosized Zn2SiO4:Mn2+ Phosphors Using the Hydrothermal Method and Their Luminescence Properties.JPhys Chem. C 114(23): 10330-10335.
- Liu B,WangY,WenY,Zhang F(2012) Photoluminescence properties of S-doped BaAl12O19:Mn2+ phosphors for plasma display panels. Mater Lett 75: 137-139.
- Izumi F, Mitomo M, Suzuki J (1982) Structure Refinement of Yttrium Alpha-Sialon from X-Ray-Powder Profile Data. J Mater Sci Lett1(12): 533-535.
- Blasse G, Bril A(1968) Fluorescence of Eu2+activated alkaline-earth aluminates.Philips Res Rep 23(2): 201-206.
- Blasse G, Wanamaker W (1968) Fluorescence of Eu+2 activated silicates. Philips Research Report 23: 189-189.
- Dexter DL (1953) A Theory of Sensitized Luminescence in Solids. J ChemPhy21(5): 836-850.
- Chung CC, Jean JH (2010) Synthesis of Ca-α-SiAlON:Eux phosphor powder by carbothermal -reduction–nitridation process. Mater Chem Phys 123(1): 13-15.
- Piao X, Machida K, Horikawa T, Hanzawa H (2008) Synthesis and luminescent properties of low oxygen contained Eu2+-doped Ca-α-SiAlON phosphor from calcium cyanamide reduction. J Rare Earths 26(2): 198-202.
- CaoCY,Yang HK,Chung JW,Moon BK (2011) Hydrothermal synthesis and enhanced photoluminescence of Tb3+ in Ce3+/Tb3+ doped KGdF4 J MaterChem 21(28): 10342-10347.
- Yang W, Luo L, Chen TM, Wang NS (2005) Luminescence and Energy Transfer of Eu- and Mn-Coactivated CaAl2Si2O8 as a Potential Phosphor for White-Light UVLED. ChemMater17(15): 3883-3888.
- You H, Zhang J, Hong G, Zhang H (2007) Luminescent Properties of Mn2+ in Hexagonal Aluminates under Ultraviolet and Vacuum Ultraviolet Excitation. The Journal of Physical Chemistry C 111(28): 10657-10661.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




