Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Mini Review(ISSN: 2641-6921) 
Isothermal Martensitic Transformation as operating Mechanism for Deep Cryogenic Treatment of Steels Volume 1 - Issue 3
Gavriljuk VG*
- Institute for Metal Physics, Ukraine
Received: March 06, 2019; Published: March 25, 2019
*Corresponding author: Gavriljuk VG, Institute for Metal Physics, Kiev, Ukraine
DOI: 10.32474/MAMS.2019.01.000112
Mini Review
Cryogenic treatment of tool steels was proposed about seventy years ago and studied more or less intensively since the fifties see e.g. [1,2]. One distinguishes between the shallow, SCT, and deep, DCT, cryogenic treatments. The first one is used at temperatures between RT and 100 C. Its effect amounts to the increase of hardness accompanied by a decrease in toughness. The second one is performed usually in the liquid nitrogen and leads to the increased wear resistance and toughness. It is remarkable that, in this case, the hardness can be even decreased. Within the last three decades, a number of results were obtained on the effect of DCT on tribological properties, e.g. [3-10]. At the same time, a definite gap exists between the scientific research and practical applications of this treatment. A reason for that is some scattering of experimental results and the absence of knowledge about phenomena taking place in the as-quenched steels cooled down to cryogenic temperatures. A number of hypotheses was proposed in the attempts to interpret the nature of a favorable DCT effect on mechanical properties of tool steels. Some of them ar based on the enhanced precipitation of ()-carbide, as it was shown by Meng et al. [3]. It is claimed, e.g. [4-6,8], that these nano-carbide particles are precipitated in the course of DCT and the following heating to RT or during subsequent low temperature tempering. At least two experimental facts are at varience with with this idea. First, the ()-carbide is found to precipitate in the high carbon martensite without any DCT. Second, this intermediate carbide is dissolved at temperatures above 200 C, whereas the tool steels are tempered at significantly higher temperatures, e.g. at 500 C. The hypotheses of “low temperature conditioning” and contraction of martensitic solid solution, see e.g., [1-8, 11-16] suppose the migration of carbon atoms towards dislocations followed by formation of nanoclucters which, in turn, serve as nucleation sites for nano-carbides. As mentioned above, no detectable migration of carbon atoms occurs in the Fe-C martensite at temperatures below 100 C. A common point in the all available hypotheses is the denial of any martensitic transformation during holding at deep cryogenic temperatures because it is believed that martensite is totally formed during cooling above 100 C. In other words, ignoring was the isothermal martensitic transformation. This transformation has been first time discovered and studied by Kurdyumov and Maximova in 1948 [17]. In contrast to the so-called athermal one characterized by the burst kinetics, it proceeds during long-time holding at temperatures below-100 ºC. As example, the isothermal martensitic transformation is shown in (Figure 1) to proceed in the tool steel during holding at-150 ºC. Pietikainen [18] was the first to find that the isothermal martensite is rather soft and acquires brittleness only starting from about 50 ºC during subsequent heating. Moreover, even the cracking accompanying the martensitic transformation in the carbon steels did not occur in his low temperature experiments.
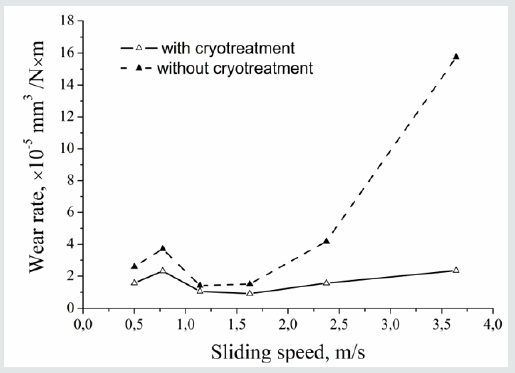
Later on, based on Mössbauer studies [19], it was shown that carbon atoms are essentially immobile in the freshly formed Fe-C martensite and its clustering occurs only during the subsequent heating. Based on the above-mentioned features [20,21], a unique phenomenon, namely the plastic deformation, has been shown to occur in the course of the isothermal martensitic transformation. Because of pinning the dislocations by mobile carbon atoms, this is impossible in case of martensitic transformation near the room temperature, RT, or in the temperature range down to -100 ºC. Sliding in the course of plastic deformation, the dislocations capture immobile carbon atoms creating carbon clouds around them. As shown in [22], due to high energy of binding between dislocations and carbon atoms in the -iron, 0.8 eV, the isothermal martensitic transformation in its optimal temperature range prevents the ()-carbide formation and shifts precipitation of cementite and special carbides to higher temperatures, causing the precipitation process incomplete. Using Mössbauer spectroscopy, it was also shown that the α solid solution in a tool steel subjected to DCT and subsequent tempering in the temperature range of secondary hardness contains complexes of carbon atoms with those of the carbide forming elements. Therefore, it contains a potential for further stress-induced precipitation in the course of the further usage of tools [22]. The results on enhancing the wear resistance with increasing sliding speed, as shown in [22], support this idea, see (Figure 2). Summing up, one should state that deep cryogenic treatment can be useful not only in case of tool steels. It is appropriate for any carbon steels with the retained austenite after quenching at RT and for any alloys where some transformations accompanied by the volume effect proceed within the range of cryogenic temperatures.
Figure 1:Isothermal martensitic transformation during holding at 150 ºC, steel 153CrMoV 12. After avriljuk et al. [22] with kind permission of Springer Science + Business Media.
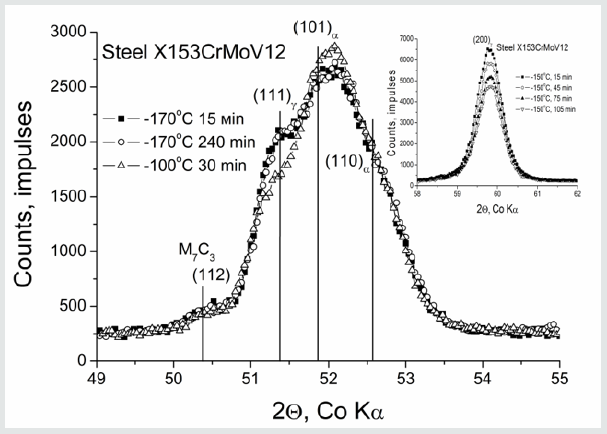
Figure 2:Wear rate vs. sliding speed for Fe–12Cr–Mo–V–1.4C tool steel subjected to austenitization at 1020 ºC and tempering at 180 ºC for 1.8ks. DCT occurred before tempering. After Meng et al. [3] with kind permission of ISIJ International.
References
- Collins DN (1996) Heat Treatment of Metals 2: 40-42.
- Barron RF (1982) Cryogenics 22(8): 409-413.
- Meng F, Tagashira K, Azuma R, Sohma H (1994) ISIJ Intern 34(2): 205- 10.
- Yen PL, Kamody DJ (1997) Industrial Heating 64(1): 40-44.
- Yun D, Xiaopin L, Hongshen X (1998) Heat Treatment of Metals 3: 55-59.
- Huang JY, Zhu YT, Liao XZ, Beyerlein IJ, Bourke MA, et al. (2003) Mat Sci & Eng A 339: 241-44.
- Bensely A, Phadhakaran A, Mohan Lal, Nagarajan G (2005) Optimization of cryogenic treatment to maximize the wear resistance of 18% Cr martensitic stainless steel by Taguchi method Cryogenics. 45: 747-54.
- Stratton PF (2007) Mat Sci & Eng A 449: 809-812.
- Baldissera P, Delprete C (2008) The Open Mechanical Engineering Journal 2: 1-11.
- Das D, Dutta AK, Ray KK (2009) Influence of Cryogenic Treatment on Microstructure and Properties Improvement of Die Steel. Wear 266: 297-309.
- Villa M, Pantleon K, Somers MAJ (2014) Acta Mater 65: 383-392.
- Chasemi Nanesa, Jahazi H (2014) Mat Sci & Eng A 598: 413-419.
- Konrade DN, Ramana KV, Jagtap KR, Dhokey NB (2017) Mater Today Proc 4: 7665-7673.
- Ciski A (1918) Arch Metall Mater 63(2): 929-934.
- Senthilkumar D (1918) Adv Mater Progr Technol.
- Li S, Min N, Li J, Wu X, Li C, et al. (2013) Mat Sci & Eng A 575: 5160.
- Kurdyumov GV, Maksimova OP (1948) Reports of Academy of Sciences of USSR in Russian 61: 83-86.
- Pietikainen J (1968) J Iron Steel Inst 206: 74-78.
- Gavriljuk VG, Gridnev VN, Nemoshkalenko VV, Razumov ON, Polushkin Yu A (1977) Physics of Metals and Metallography 43(3): 582-590.
- Tyshchenko AI, Theisen W, Oppenkowski A, Siebert S, Razumov ON, et al. (2010) Mat Sci Eng A 527: 7027-7039.
- Gavriljuk G, Theisen W, Sirosh VV, Polshin EV, Kortmann A (2019) Acta Mater 61: 1705-1715.
- Gavriljuk VG, Sirosh VA, Petrov Yu N, Tyshchenko AI, Theisen W (2014) Metall and Mater Trans A 45(5): 2453-2465.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




