Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5787
Review Article(ISSN: 2637-4692) 
Role of Adiponectin in Immunity; Implications for Infectious Diseases and Cancer Volume 1 - Issue 3
Sze Ping Tan1, Alexander David Saleh2 and Peng Hong Tan2*
- 1GKT School of Medical Education, King’s College London, University of London, UK
- 2Department of Plastic and Reconstructive Surgery, Royal Free NHS Foundation Trust, University College of London, UK
Received:September 10, 2019; Published:September 17, 2019
Corresponding author:Peng Hong Tan, Department of Plastic and Reconstructive Surgery, Royal Free NHS Foundation Trust, University College of London, UK
Abstract
Adipose tissues are not only simply dormant storage depots, but they are dynamic and highly active organs. One of soluble molecules generated by adipocytes is adiponectin (APN). It has been shown to have direct anti-diabetic and anti-atherogenic properties. The role of APN in immunity has been subjected to many controversies lately. Therefore, this review addresses all controversies associated with the effect of APN on the immunity critically. Overall, the evidence supports that APN receptor signaling on the immune cells can promote immune regulation and induce antigen-specific tolerance. This may have significant implications for treatment of many diseases includes cancer, inflammatory related conditions and infectious diseases.
Keywords:Adiponectin; AdipoR1 and AdipoR2; Adaptive immunity and Innate Immunity
Introduction
Adipocyte-related metabolic pathways are crucial in controlling the immune system. For example, pro-inflammatory cytokines are often over-expressed in obesity [1]. In diet-induced obese insulinresistant mice, their adipose tissues were also infiltrated with T cells [2,3]. Progressive fat cell enlargement and disposition in obesity, leading to a hypoxic microenvironment, can also further promote a localized inflammatory state. In addition, they also produce many secretory molecules termed as adipokines which can affect the immune system. Of these adipokines, adiponectin (APN) has been well-characterised for its impact on the immune cells.
Thus far, majority of evidence support that APN can modulate the immune system mainly via inhibiting the synthesis of pro-inflammatory cytokines and the induction of many antiinflammatory cytokines by antigen presenting cells (APC). Indeed, APN-deficient mice had higher levels of, pro-inflammatory cytokine, tumor necrosis factor (TNF)- α than wild-type counterparts [4]. However, other studies have suggested contrary observations. These may due to many factors and experimental conditions. For example, the kinetics of APN receptor (either AdipoR1 or AdipoR2) expression, are different for different immune cells or an immune cell at its different stages of its differentiation. AdipoR1 is more abundant than AdipoR2 in monocytes of the innate immunity and its expression decreases upon their differentiation into macrophages, whereas AdipoR2 remains constant [5]. Only 10% of T cells in the adaptive immunity express AdipoR1/R2 compared to 80-90% of CD14+ cells (including monocytes) [6]. As these receptors have different and separable signaling pathways, it can then generate a complex outcome depending on the stage where and when these receptors are activated. Furthermore, the insulin receptors can inversely affect the sensitivity of APN receptors [7]. Therefore, the experimental results on the effect of APN on the immune system needs to be interpreted cautiously.
Adding to the complexity, the pleiotropic effects of APN are also influenced by the presence of its various oligomers. For example, oligomers, such as hexamers, may act different signaling pathways in different anatomical sites, resulting in different physiological responses [8,9]. Whereas at the ligand-receptor level, one study indicates that only the full-length APN (flAcrp) can upregulate proinflammatory cytokine production at the protein level, but not at its mRNA levels, whereas the globular APN (gAcrp) transiently induces its production at the protein and mRNA levels [10]. However, this then renders immune cells resistant to further pro-inflammatory stimuli via suppression of its scavenger receptor and induction of IL-10 production [10]. High molecular weight (HMW) APN requires a shorter incubation period to inhibit the nuclear factor-kappa B (NF-κB) pathway [11], compared with gAcrp. All observations can sometimes make the definition of function for APN in the immune system more challenging. In this review, we explore complexity of APN effect on the immune system.
Adiponectin and its receptors
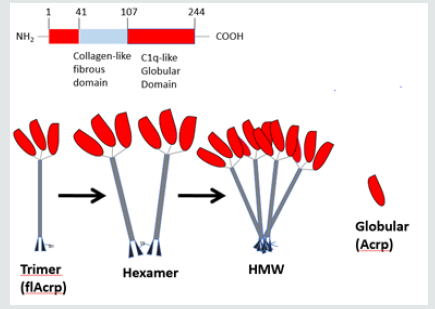
APN is encoded by the ADIPOQ gene on chromosome locus 3q27, which has been implicated in development of metabolic syndrome [12]. The expressed protein shares homologies with collagen with a structure that is not dissimilar to the complement factors, TNFα and the brain specific factor cerebellin. The full-length protein (flAcrp) primary structure contains a N-terminal signal sequence, a variable region, a collagen-like domain and a C-terminal domain (Figure 1). In some cases, this monomeric flAcrp trimerizes to form the Low Molecular Weight (LMW) isoform. This LMW isoform can also form hexamers (MMW) or 12- and 18-mers (HMW) via disulphide bonds (Figure 1). This HMW version appears to be the most biologically active isoform and has the highest plasma concentrations. The monomeric version appears to be confined to the adipocytes, whereas the oligomeric isoforms are present in the circulation [13]. Via photolytic cleavage of globular C terminal domain of flAcrp, a globular form (gAcrp) is formed.
These isoforms may impose different physiological effects and may also account for some of the pleiotropic effects exerted by APN [8,9]. The normal serum concentration of APN is 2-30μg/ml. The levels can be influenced by several factors including; genetics [14], diet [15], various pharmacological agents [16,17], inflammatory status [18], hypoxia [19], exercise [20], renal function [21], age [21] and sex hormones [21].
3 different receptors have been identified to bind to various isoforms of APN; namely AdipoR1 [22], AdipoR2 [22] and the more recently T-cadherin [23]. AdipoR1 has a higher affinity for gAcrp than flAcrp and is abundantly expressed in skeletal muscle. In contrast, in the liver, AdipoR2 is predominantly expressed, and its affinity for both gAcrp and flAcrp has determined to be intermediate to low. T-cadherin, encoded on the Cadherin-13 (CDH13) gene, has been proven to interact HMW and hexametric APN [24]. Its actions are crucial for cell adhesion and calcium-mediated cell-cell interactions and signaling, acting via the 5’ Adenosine monophosphate (AMP)- activated protein kinase (AMPK) pathway. This pathway is involved in cellular energy homeostasis.
Role of APN in innate immunity
APN can affect immunity indirectly via its action of the immune cells. Both professional antigen-presenting cells (APC) such as the monocytes, macrophages and dendritic cells and non-professional antigen cells such as endothelial cells can be influenced by the treatment of APN. Its effects can be variable depending on the experimental conditions and treatment doses. In addition to its effect on immune cells, gAcrp can directly bind to chemokines such as SDF-1, CCF18, MIP1a, RANTES and MCP-1, in order to neutralize its immunological effects [25].
a) Monocytes and macrophages
APN effects on the innate immune system are primarily mediated via its inhibition of NF-κB pathway [26-29]. In monocytes, it has been shown to induce production of anti-inflammatory cytokine such as IL-10 and IL-1RA [30]. Similarly, in macrophages, treatment of cells with APN induces various anti-inflammatory cytokines, such as IL-10 [10,30-40], IL-1RA [30] and IL-4 [40]. Some groups have gone on to show that this anti-inflammatory cytokine production can modulate disease process via various mechanisms. Not only the function of macrophages is affected by treatment of APN, the cellular maturation of cell can also be influenced. APN directly suppresses maturation of macrophages hence affecting its function [41]. Many groups have ever gone on to show that an exposure to APN can impair the pro-inflammatory cytokine production by macrophages following its pro-inflammatory stimulation. For example, IL- 1β(32), IL-6 (10, 31) and TNF-α [22,32,37,38,40] productions are diminished following the activation of macrophages. Not only these cytokines are modulated, chemokine secretion such as IP10/ CXCL10, I-TAC/CXCL11, Mig/CXCL9 and CCL18 can be affected as well [42].
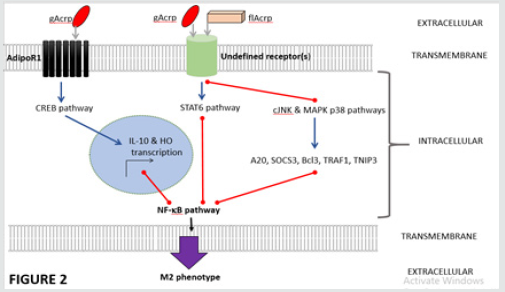
Impairment of pro-inflammatory phenotypes of macrophages is due to the suppression of its NFκB pathway via the inhibitions of extracellular-signal-regulated kinase (ERK) 1/2 and mitogenactivated protein kinase (MAPK) p38(31), c-Jun Kinase/stress activated (c-JNK) and MAPKp38 [27], and signal transducer and activator of transcription 3 (STAT3) [27] pathways (Figure 2). Some groups have proposed that the NFκB suppression may due to the activation of AMPK pathway [32]. The production of anti-inflammatory cytokines by macrophage is postulated due to induction of cAMP response element-binding protein (CREB)- mediated transcription of IL-10 through activation of AMPK [39, 40] and ERK1/2 [39] pathways when gAcrp interacts with AdipoR1 [40]. Whereas, the flAcrp engagement with its receptor can activate STAT6-mediated IL-4 transcription [40]. Hence, the transcript some resembling M2 (anti-inflammatory phenotype) rather than M1 was observed following APN treatment on macrophages [43] (Figure 2). In disease models, less immune cellular infiltration was noted [32, 44]. This is due to APN activates macrophage autophagy (via adenosine 5’-monophosphate-activated protein kinase pathway) and stimulates endothelial nitric oxide synthase (eNOS) pathway. In many instances, early apoptotic bodies can be pro-inflammatory, and APN can promote calreticulin receptor-dependent clearance of these bodies [45]. Therefore, these directly influence the function of macrophages therefore affecting the immuno-reactivity.
Having said that, others have reported that APN can induce a transient pro-inflammatory cytokine production [33,46,47]. For example, TNF-α [10,33,38] and IL-6 [10,48] production can be induced following treatment of macrophages with APN. These cytokine productions can then be subsequently suppressed by antiinflammatory cytokine, IL-10 which is also induced by APN. The clear mechanistic action on how the APN induce pro-inflammatory cytokine production remains elusive. One has proposed that gAcrp transiently activates ERK-1/2 pathway, which in turn activates early growth response protein-1 (also known as Zif268 (zinc finger protein 225)) (Egr-1) and then this triggers NFκBdependent mechanism [33,38]. However, subsequently the NFκB pathway is suppressed by APN-induced IL-10 production [33,38]. Alternatively, another group proposes that APN can activate the NFκB pathway via phospholipase C (PLC)- gand c-Jun pathways [46]. Past work on the signaling pathways has challenged this notion indicating that the NFκB-mediated response is independent of JNK pathway, but it is instead dependent on AMPK, MAPK p38 and ERK1/2 pathways [49]. APN can trigger the STAT-3 pathway to upregulate insulin receptor substrate (IRS)-2 to activate NFκB mechanism; in order to produce IL-6. The authors have argued that this process is independent of APN engagements with its cognate receptors, AdipoR1/R2 [48]. Therefore, this activation supports that induction of transcript some mimicking pro-inflammation [47]. One can argue that the experimental conditions are suboptimal resulting in the contradictory observation. In this case, the dose of APN used to treat the cells was suboptimal.
b) Dendritic cells
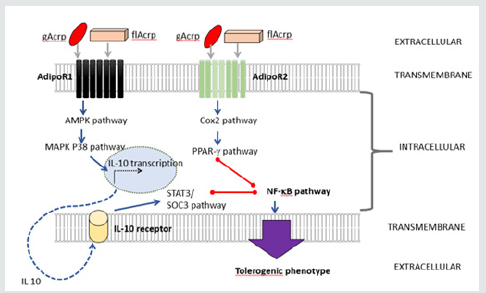
In dendritic cells, pre-treatment with APN can induce antiinflammatory cytokine; IL-10 [30,50] and IL-1RA [30]. When cells were treated at early stage of maturation, these cells were arrested at the immature state as following activation with pro-inflammatory stimuli, they failed to develop to the matured phenotypes [50,51]. The cells fail to upregulate positive co-stimulators (CD80 [50,51], CD86 [50,51] and CD40 [50]) and they also directly downregulate human leukocyte antigen (HLA)/major histocompatibility complex (MHC) class II [50,51]. These render the cells less able to present antigens to the adaptive immunity. Another group has reported that there was a direct upregulation of negative co-stimulator (PD1-PDL1 [51]), that could further affect its antigen presenting ability. In order define the mechanistic action, it has been shown in dendritic cells that interaction of either globular or full-length forms with AdipoR1, signals via its conventional pathways using AMPK
and MAPKp38 to upregulate IL-10 [50] (Figure 3). This in turn acts in an autocrine or perhaps paracrine manner to promote its downstream pathways [50]. Activation of IL-10 receptor signaling triggers the STAT3/Suppressor of cytokine signaling (SOCS)-3 pathway [50]. The STAT3 signaling pathway is far more confusing; due to, the fact that some pro-inflammatory cytokines such as IL-6 uses this pathway to signal [52,53]. However, in a system with two opposing cytokines signaling simultaneously, some work proposes that the action of IL-10 (anti-inflammatory) may supersede that of IL-6 (pro-inflammatory) [54]. A recent work on conditioning STAT3-knockout-dendritic cells demonstrated that STAT3 is crucial in mediating IL-10-dependent tolerance [55]. Indeed, AdipoR1- signalling in dendritic cells mediates IL-10-dependent T-cell tolerance in the manner that it is relied on STAT3 activation [50]. Although STAT3/SOCS3 pathway does appear to be the dominant mediator of IL-10’s functions, there are elements of its antiinflammatory activity that may be STAT3/SOCS3-independent [56]. For example, STAT3 activation by IL-10 receptor signaling can lead to the activation of alternative downstream molecules such as an E26 transformation specific (ETS)-family transcriptional repressor, ETV3, and Strawberry notch homologue 2 (SBNO2), that in turn, can repress NF-κB activation [56].
In AdipoR1-depleted dendritic cells, AdipoR2 triggers the COX2 pathway and subsequently it is responsible for the upregulation of peroxisome proliferator-activated receptor-γ (PPARγ)which then acts to promote energy on its encountering T cells; as knockout of PPARγ abolishes this phenotype [50]. This is consistent with the previous work showing that stimulation of PPARγ with its agonist has been identified to be responsible for the tolerogenic behaviors of APC [57]. A recent work indicates that AdipoR2-signalling following its engagement with either globular of full-length forms, via the PPARγ pathway, predominantly drives Th2 cytokines by the encountering T cells [50]. This is also consistent with the experiments in which APCs were treated with a PPARγ agonist [58]. Greater Th2-skewing with AdipoR2-signaling is observed than in the case of signaling through AdipoR1 [50]. Significantly more Th2- skewing cytokine production was noted when the dendritic cells possess dominant AdipoR2 signaling [50]; but a small degree of Th2-skewing noted on the AdipoR1-signalled dendritic cells may be due to the SOCS3 effects that was induced by AdipoR1 [59]. Having said that, some groups have reported that pre-treatment cells with APN can activate the cells and fasten the maturation process with hastening the expression positive co-stimulators (CD86 and CD40) and up-regulating HLA II [46]. An induction of production of proinflammatory cytokines; IL-12, IL-6, IL-1β and IL-23 was observed [46]. This contradictory observation may again due to the fact the dose of APN used in these experimental conditions is suboptimal to suppress the cells.
c) No professional antigen presenting cells (APC)
In endothelial cells, a non-professional APC; treatment of APN can inhibit adhesion molecule expression such as intracellular adhesion molecule (ICAM)-1 (CD54) [60] and E-selectin (CD62E) [61] and can impair the production of soluble vascular endothelial growth factor receptor 1 (sVEGFR1) [61]. These effects result in less infiltration of immune cells into disease models [42,62]. Suppression of NF-κB via the stimulation of AMPK pathway which in turn induces CCAAT-enhancer-binding protein (C/EBP) α [63] (independent of ERK1/2, MAPKp38, c-JNK) [63] has been heralded as a central mechanism.
In addition, the ability of the endothelial cells to generate proinflammatory cytokine; TNF-α [64, 65], IL-6 [26,61] and IL-8 [64] were also directly suppressed. Modulation of pro-inflammatory cytokine production by endothelial cells [65] may be due to CRT/ CD91-mediated COX2 activation [66]. Alternatively, inhibition of vascular inflammation may also be due to Cavolin-1-mediated ceramidase pathway (only via AdipoR1 has been shown) [60]. Pre-treatment of endothelial cells with APN also inhibits cellular apoptosis and modulates ROS production via its direct activation of AMPK pathway [65,67] that induces eNOS [44,68,69]. Activating AMPK (also inhibiting ERK1/2) [70] has shown to inhibit hypertrophic signals via T-cadherin [23]. Less subtle effect on endothelial progenitor cells (EPC) function and its localization can be observed following the exposure to APN [71]. All these render the endothelial cells less inflammatory to the adaptive immunity.
d) Natural killer cells
APN has also been reported to negatively regulate Natural Killer (NK) cell function in the IL-2–stimulated model [72]. APN was found to regulate AMPK-mediated inhibition of NFκB activation, and subsequently to down-regulate the IFNγ-inducible TNF-related apoptosis-inducing ligands (TRAIL) and Fas ligand expression on NK cells [72]. This directly modulates the function of NK cells.
Role of APN in adaptive immunity
In addition to the effect on APN on the innate immunity, evidence also suggest the APN can also influence the adaptive immunity mainly T cells and B cells.
a) T cells
Effector T cells (Th1 and Th17) and regulatory T cells can be affected by APN. Some groups suggest that APN may act as a negative regulator of T cell proliferation [6]. Only a small percentage of T cells express the AdipoR1/R2 on their surface, but most of these receptors are stored in the clathrin-coated vesicles that are found to be co-localized with the T cell negative co-stimulators. This implicates it may control on T cell expansion following its activation [6]. Early in vitro experiments suggest that APN treatment inhibits antigen-specific T cell proliferation [6,51] and suppresses the pro-inflammatory cytokine; IL-1 [50], IL-2 [6], IL-8 [50], TNF-α [6], IFN-γ [6,50]. In vivo, T cell apoptosis induced by APN may be central to the process how APN can control the adaptive immunity [6]. For example, in an infective model, inoculation with APN generates less effector T cells in wild-type animal, whereas, in APNknockout mice, this inoculation generates more effector T cells [6]. Furthermore, effector T cells that are activated by APN-treated APC are rendered anergic either in vitro or in vivo [50]. On the other hand, APN treatment could also selectively promote the expansion of regulatory T cells [51], which may further control the immune responses. Differential response of effector T cells and regulatory T cells to APN may explain how APN regulates the adaptive immunity.
Not only does APN directly affect effector T cells or regulatory T cells, but the APN has been shown to affect the migration of T cells into the disease site. For example, in an atherogenic model, APN can reduce T cell recruitment and accumulation during atherogenesis via modulation of chemokine production by the resident macrophages [42]. Similarly, in the cardiac allograft transplantation model, APN-deficient mice showed a severe acute rejection, manifested by an increase in T cells and macrophage infiltration into the rejected organs [62].The may be indirectly due to the induction of reactive T cell apoptosis via induction of caspase [6] or perhaps due to T cell energy [50]. Of interest, there is one study reported that APN can activates T cells via induction of INF-γ and IL-6 [47] and subsequently promotes Th1 and Th17 differentiation [46]. Augmenting T-bet expression via activation of MAPKp38 and STAT4 pathways [47] has proposed as a possible mechanistic pathway [46]. This notion pales into insignificance as majority reports shown otherwise. Moreover, the treatment dose used in this contradictory report was also suboptimal.
b) B cells
Work has shown that APN inhibits B lymphopoiesis [41]. APN caused elevated expression of COX-2 by these stromal cells and induced release of prostaglandin E (2) (PGE (2)) [73]. APN can negatively and selectively influence lymphopoiesis through induction of PG synthesis [41].
Conclusion
On face value, the effect of APN on the immunity may appear to be contradictory, but when one carefully scrutinizes findings and experimental protocols, these contradictions can be explained [74,75]. For example, one group has indicated that APN can induce an activation of NFκB which polarizes the immune reaction towards a pro-inflammatory response [46]. However, this report pales into insignificance because many groups have shown completely opposite findings in human [10,30,35,43,50,51], murine [38,50,76], porcine [31] or rodent [40]-derived APCs. In fact, one group has previously claimed that APN triggers a multifaceted response in macrophages by inducing the expression of various anti-inflammatory proteins that act at different levels in concert to suppress its activation [27]. They went to confirm that APN inhibits phosphorylation of NFkB via its inhibition of the upstream MAPKp38, JNK and STAT-3 pathways [27]. However, surprisingly, the same group has now shown that APN can induce pro-inflammatory functions in isolated macrophages and T cells, hence inducing a limited program of inflammatory activation and they claim that this activation likely desensitizes these cells to further pro-inflammatory stimuli [47]. It is important to note that all these controversial studies showing the pro-inflammatory effect of APN are using APN concentrations at the sub-optimal level (at 10 microgram/ml which is just a normal physiological concentration in serum) to condition the immune cells [46,47].
In a non-obese and non-diabetic person, 10mg/ml is often defined as the average level for APN [77]. Using this physiological level (at 10 microgram/ml) [46,47] to condition the immune cells and subsequently to determine the immune outcome could be argue as a sub-optimal way to define its role. In many systems, it has reported that when the substrate used to condition the immune cells are sub-optimal, it can generate the opposite effects. For example, when anti-oxidative vitamins such as vitamin C or E were used to condition the dendritic cells at the sub-optimal level, they result in pro-inflammatory reactions, instead of having an antiinflammatory effect [78]. If the doses of vitamins were deployed at higher levels, they exhibit its “true” anti-oxidative property and modulate the immune response towards an anti-inflammatory state [78]. This argues that it is crucial to use an optimal concentration of APN to study its intended effects. Indeed, the latest study may explain the contradiction in the recent papers; it has been shown that at least 4 times of the normal APN plasma level (at 40 microgram/ml) is required to induce the IL-10 production in the immune cells, resulting in its intended anti-inflammatory effects [50]. A 400% requirement does not seem to be excessive in this case, as the physical exercise alone can induce a 260% increase in serum APN [79]. Therefore, it is illogical for the previous work to ascertain the effect of APN at normal physiological level (10 microgram/ml) on the immune system and then define its function. It is likely at this level; the APN receptors are not fully saturated and its subsequent signaling is likely to be sub-optimal and so then the intended outcome can sometimes be misleading. Biologically, as serum APN fluctuates greatly with mild activities, it is therefore not surprising that higher doses of APN will be required to saturate its receptors [80], otherwise the human physiology will be extremely unstable.
It is universally accepted that APN can induce IL-10 transcription [10,30-40,50] and most work have confirmed that gAcrp is probably the best effector for induction of IL-10 production. However, the mechanism on how IL-10 transcription is induced may be variable, as earlier work did not directly study the cognate interaction of APN with its paired receptor. It has proposed that the initial transient TNF-α/IL-6 production, following engagement of APN with unidentified receptor(s), activates ERK1/2, then the Egr-1 and NFκB pathways [33,38]. This may be crucial for IL-10 transcription. Conventionally, it has been shown that IL-10 transcription is mediated by phosphorylation of CREB and the cAMP response element in the IL-10 promoter that triggers its transcription [34,38]. Indeed, this CREB-mediated IL-10 transcription is activated through the AMPK and ERK1/2 pathways following the exposure to gAcrp [76]. Recently one group has elegantly demonstrated that interaction of either gAcrp or flAcrp with AdipoR1, signals its conventional pathways using AMPK and MAPKp38 to upregulate IL-10. This in turn acts in an autocrine or perhaps paracrine manner to promote its downstream pathways [50]. Activation of IL-10 receptor signaling triggers the STAT3/ Suppressor of cytokine signaling (SOCS)-3 pathway [50] that triggers inhibition of NFκB pathway.
APN interaction with its cognate receptors is more far complex. In models where IL-10-mediated and PPARγ-mediated T cell tolerance due to AdipoR1 and AdipoR2 signaling respectively, it is plausible that induction of various anti-NFκB effectors may have contributory effects in promoting the APC tolerogenic phenotypes [50]. In the case of AdipoR1-signaling, A20 (zinc finger protein) and B-cell lymphoma 3-encoded protein (Bcl3) (a greater upregulation following AdipoR1-signaling) may counter-inhibit the proinflammatory action of IL-6 that previously has been implicated to be associated with APN signaling [27,50]. Inhibiting these anti- NFκB effectors does not reverse the ability of APN-conditioned APCs to promote T-cell energy (in single receptor depleted system) [50]. This indicates these molecules act collaboratively with either IL-10 or PPARγ to mediate anti-inflammatory effects [50]. So far, in the immune system, no work has directly addressed the effects of APN’s interaction with T-cadherin, which has been implicated to mediate cardio-protection via the AMPK pathway [23]. However, in the double receptor depleted system (both AdipoR1 and AdipoR2), reversal of APN-induced T-cell energy was noted [50]; indicating APN-signaling via T-cadherin or other unidentified receptor(s) is unlikely to mediate T-cell tolerance.
On the balance of all probabilities, APN is likely to affect both innate and adaptive immunity negatively causing the induction of immune tolerance and anti-inflammatory state. This antiinflammatory effect may be beneficial in treatment of auto-immunity, transplant rejection and cardiovascular diseases. Development of its agonist and inducer for APN expression may be helpful in these conditions. On the other hand, APN may also hamper the ability to fight against various infectious agents. Therefore, ability to module its expression in the context of where is a need to mount a strong immune response to fight active infection need to be explored. Arguably, the development of its antagonist may aid the success of treatment of chronic infection. Similarly, understanding of its role in the immunity may help us to harness its function in designing an effective oncological treatment that requires anti-tumor immunity.
Figures and Legends
Figure 1 A depicts a structure APN from N terminal to C-terminal; B shows various oligomers of APN, LMW: Low molecular weight. MMW: Middle molecular weight. HMW: Heavy molecular weight. flAcrp: Full length adiponectin. Acrp: Globular adiponectin. Figure 2 Through AdipoR1 and undefined receptors APN causes NFκB inhibition and promotion of M2 phenotype. BLUE arrows represent activating pathways. RED lines represent inhibitory pathways. gAcrp: globular adiponectin. flAcrp: full length adiponectin. STAT 6: Signal transducer and activator of transcription 6. CREB: cAMP response element-binding protein. JNK: c-Jun N-terminal kinase. SOC3: Suppressor of cytokine signaling 3. Bcl3: B-cell lymphoma 3-encoded protein. TRAF1: TNF receptor-associated factor 1. TNIP3: TNFAIP3-interacting protein 3. NF -κB: nuclear factor kappa-lightchain- enhancer of activated B cells. Figure 3 APN generates dendritic cell tolerance via two distinct pathways that converge upon NF-kB inhibition. BLUE arrows represent activating pathways. RED lines represent inhibitory pathways. gAcrp: globular adiponectin. flAcrp: full length adiponectin. AMPK: 5’ APM activated protein kinase. Cox2: cyclooxygenase 2. PPAR-γ: Peroxisome proliferator-activated receptor gamma. NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells. STAT 3: Signal transducer and activator of transcription 3. SOC3: Suppressor of cytokine signaling 3.
Declarations
a) Acknowledgments
Due to the space restrictions, the authors were only able to cite a fraction of the relevant literature. We apologize to any colleagues whose contribution might not be appropriately acknowledged in this review.
b) Authors’ contributions
SPT and AS contributed toward the preparation of the manuscript. PHT provided guidance and mentorship in writing and revising the manuscript.
c) Availability of data and materials
Not applicable.
d) Financial support and sponsorship
PHT is funded by Royal Free NHS Foundation Trust as a fulltime Consultant Oncoplastic Breast Surgeon.
e) Conflicts of interest
All authors declared that there are no conflicts of interest.
f) Ethical approval and consent to participate
Ethical approval not required.
g) Consent for publication
Not applicable.
References
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, et al. (1995) The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95(5): 2111-2119.
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, et al. (2007) T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115(8): 1029-1038.
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15(8): 914-920.
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, et al. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8(7): 731-737.
- Chinetti G, Zawadski C, Fruchart JC, Staels B (2004) Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors Paraph, PPARgamma, and LXR. Biochem Biophys Res Commun 314(1): 151-158.
- Wilk S, Scheibenbogen C, Bauer S, Jenke A, Rother M, et al. (2011) Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol 41(8): 2323-2332.
- Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, et al. (2004) Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279(29): 30817-30822.
- Wang Y, Lam KS, Xu JY, Lu G, Xu LY, et al. (2005) Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280(18): 18341-18347.
- Palanivel R, Fang X, Park M, Eguchi M, Pallan S, et al. (2007) Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovascular research 75(1): 148-157.
- Kyriazi E, Tsiotra PC, Boutati E, Ikonomidis I, Fountoulaki K, et al. (2011) Effects of adiponectin in TNF-alpha, IL-6, and IL-10 cytokine production from coronary artery disease macrophages. Horm Metab Res 43(8): 537-544.
- Tomizawa A, Hattori Y, Kasai K, Nakano Y (2008) Adiponectin induces NF-kappaB activation that leads to suppression of cytokine-induced NF-kappaB activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diab Vasc Dis Res 5(2): 123-127.
- Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, et al. (2000) Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA 97(26): 14478-14483.
- Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH (2007) The oligomeric structure of high molecular weight adiponectin. FEBS Lett 581(5): 809-814.
- Arikoglu H, Ozdemir H, Kaya DE, Ipekci SH, Arslan A, et al. (2013) The Adiponectin Variants Contribute to the genetic background of type 2 diabetes in Turkish population. Gene 534(1): 10-16.
- Silva FM, de Almeida JC, Feoli AM (2011) Effect of diet on adiponectin levels in blood. Nutr Rev 69(10): 599-612.
- Blanco Colio LM, Martín Ventura JL, Gómez Guerrero C, Masramon X, de Teresa E, et al.
(2008) Adiponectin plasma levels are increased by atorvastatin treatment in subjects at high cardiovascular risk. Eur J Pharmacol 586(1-3): 259-265. - Kohlstedt K, Gershome C, Trouvain C, Hofmann WK, Fichtlscherer S, et al. (2009) Angiotensin-converting enzyme (ACE) inhibitors modulate cellular retinol-binding protein 1 and adiponectin expression in adipocytes via the ACE-dependent signaling cascade. Mol Pharmacol 75(3): 685-692.
- Lee YJ, Cho S, Kim SR (2011) The association between serum adiponectin levels and nutritional status of hemodialysis patients. Ren Fail 33(5): 506-511.
- Zappala G, Rechler MM (2009) IGFBP-3, hypoxia and TNF-alpha inhibit adiponectin transcription. Biochem Biophys Res Commun 382(4): 785-789.
- Saunders TJ, Palombella A, McGuire KA, Janiszewski PM, Després JP, et al. (2012) Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab 2012: 148729.
- Isobe T, Saitoh S, Takagi S, Takeuchi H, Chiba Y, et al. (2005) Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol 153(1): 91-98.
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941): 762-769.
- Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz Lozano P, et al. (2010) T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest 120(12): 4342-4352.
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, et al. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101(28): 10308-10313.
- Masaie H, Oritani K, Yokota T, Takahashi I, Shirogane T, et al. (2007) Adiponectin binds to chemokines via the globular head and modulates interactions between chemokines and heparan sulfates. Exp Hematol 35(6): 947-956.
- Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, et al. (2012) Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol 188(2): 854-863.
- Folco EJ, Rocha VZ, Lopez Ilasaca M, Libby P (2009) Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem 284(38): 25569-25575.
- Lira FS, Rosa JC, Pimentel GD, Seelaender M, Damaso AR, et al. (2012) Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-kappaB pathway in 3T3-L1 adipocytes. Cytokine 57(1): 98-106.
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, et al. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100(25): 2473-2476.
- Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323(2): 630-635.
- Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME (2004) Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316(3): 924-929.
- Qi GM, Jia LX, Li YL, Li HH, Du J (2014) Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology 155(6): 2254-2265.
- Park PH, McMullen MR, Huang H, Thakur V, Nagy LE (2007) Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem 282(30): 21695-21703.
- Luo N, Wang X, Chung BH, Lee MH, Klein RL, et al. (2011) Effects of macrophage-specific adiponectin expression on lipid metabolism in vivo. Am J Physiol Endocrinol Metab 301(1): E180-E186.
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, et al. (2004) Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109(17): 2046-2049.
- Kollias A, Tsiotra PC, Ikonomidis I, Maratou E, Mitrou P, et al. (2011) Adiponectin levels and expression of adiponectin receptors in isolated monocytes from overweight patients with coronary artery disease. Cardiovasc Diabetol 10:14.
- Kamio N, Akifusa S, Yamaguchi N, Nonaka K, Yamashita Y (2009) Anti-inflammatory activity of a globular adiponectin function on RAW 264 cells stimulated by lipopolysaccharide from Aggregatibacter actinomycetemcomitans. FEMS Immunol Med Microbiol 56(3): 241-247.
- Huang H, Park PH, McMullen MR, Nagy LE (2008) Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol 23 Suppl 1: 50-53.
- Park PH, Huang H, McMullen MR, Bryan K, Nagy LE (2008) Activation of cyclic-AMP response element binding protein contributes to adiponectin-stimulated interleukin-10 expression in RAW 264.7 macrophages. J Leukoc Biol 83(5): 1258-1266.
- Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE (2011) Molecular mechanism for adiponectin dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 286(15): 13460-13469.
- Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, et al. (2003) Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol 171(10): 5091-5099.
- Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, et al. (2008) Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 102(2): 218-225.
- Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, et al. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285(9): 6153-6160.
- Zhang C, Liao Y, Li Q, Chen M, Zhao Q, et al. (2013) Recombinant adiponectin ameliorates liver ischemia reperfusion injury via activating the AMPK/eNOS pathway. PLoS One 8(6): e66382.
- Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, et al. (2007) Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117(2): 375-386.
- Jung MY, Kim HS, Hong HJ, Youn BS, Kim TS (2012) Adiponectin induces dendritic cell activation via PLCgamma/JNK/NF-kappaB pathways, leading to Th1 and Th17 polarization. J Immunol 188(6): 2592-2601.
- Cheng X, Folco EJ, Shimizu K, Libby P (2012) Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem 287(44): 36896-36904.
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, et al. (2011) Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab 13(4): 401-412.
- Fan D, Li L, Wang C, Cui XB, Zhou Y, et al. (2011) Adiponectin induces interleukin-6 production and its underlying mechanism in adult rat cardiac fibroblasts. J Cell Physiol 226(7): 1793-1802.
- Tan PH, Tyrrell HE, Gao L, Xu D, Quan J, et al. (2014) Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res 74(20): 5711-5722.
- Tsang JY, Li D, Ho D, Peng J, Xu A, et al. (2011) Novel immunomodulatory effects of adiponectin on dendritic cell functions. International immunopharmacology 11(5): 604-609.
- Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC (2001) Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA 98(16): 9014-9019.
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, et al. (1998) Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161(9): 4652-4660.
- Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, et al. (2003) Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol 170(6): 3263-3272.
- Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, et al. (2010) Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol 184(5): 2638-2645.
- Williams L, Bradley L, Smith A, Foxwell B (2004) Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol 172(1): 567-576.
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, et al. (2007) Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol 178(4): 2122-2131.
- Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, et al. (2001) Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol 31(10): 2857-2865.
- Li Y, Chu N, Rostami A, Zhang GX (2006) Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol 177(3): 1679-1688.
- Wang Y, Wang X, Lau WB, Yuan Y, Booth D, et al. (2014) Adiponectin inhibits tumor necrosis factor-alpha-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ Res 114(5): 792-805.
- Van Meurs M, Castro P, Shapiro NI, Lu S, Yano M, et al. (2012) Adiponectin diminishes organ-specific microvascular endothelial cell activation associated with sepsis. Shock 37(4): 392-398.
- Okamoto Y, Christen T, Shimizu K, Asano K, Kihara S, et al. (2009) Adiponectin inhibits allograft rejection in murine cardiac transplantation. Transplantation 88(7): 879-883.
- Yu F, Chen R, Takahashi T, Sumino H, Morimoto S, et al. (2008) Candesartan improves myocardial damage in obese mice with viral myocarditis and induces cardiac adiponectin. Int J Cardiol 129(3): 414-421.
- Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, et al. (2005) Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res 97(12): 1245-1252.
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, et al. (2005) Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11(10): 1096-1103.
- Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, et al. (2009) Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol 29(13): 3487-3499.
- Zhi Z, Pengfei Z, Xiaoyi T, Genshan M (2014) Adiponectin ameliorates angiotensin II-induced vascular endothelial damage. Cell Stress Chaperones 19(5): 705-713.
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, et al. (2004) Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279(2): 1304-1309.
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, et al. (2006) Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47(6): 1108-1116.
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, et al. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10(12): 1384-1389.
- Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, et al. (2008) Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett 582(11): 1607-1612.
- Kim KY, Kim JK, Han SH, Lim JS, Kim KI, et al. (2006) Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol 176(10): 5958-5964.
- Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, et al. (2002) Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J Clin Invest 109(10): 1303-1310.
- Katira A, Tan PH (2015) Adiponectin and its receptor signaling: an anti-cancer therapeutic target and its implications for anti-tumor immunity. Expert Opin Ther Targets 19(8): 1105-1125.
- Monks M, Irakleidis F, Tan PH (2019) Complex interaction of adiponectin-mediated pathways on cancer treatment: a novel therapeutic target. J Cancer Metastasis Treat 5(24).
- Park PH, Huang H, McMullen MR, Mandal P, Sun L, et al. (2008) Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem 283(40): 26850-26858.
- Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, et al. (2003) Plasma levels of adiponectin are associated with insulin resistance and serum levels of triglyceride in Japanese metabolically obese, normal-weight men with normal glucose tolerance. Diabetes Care 26(10): 2964-2965.
- Tan PH, Sagoo P, Chan C, Yates JB, Campbell J, et al. (2005) Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol 174(12): 7633-7644.
- Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, et al. (2004) Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care 27(2): 629-630.
- Yamauchi T, Iwabu M, Okada Iwabu M, Kadowaki T (2014) Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 28(1): 15-23.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...