
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Review Article(ISSN: 2637-4544) 
Probiotics Intervention in Woman Health: Unexpected Acquaintance Volume 2 - Issue 1
Krishna Suresh Babu Naidu*
- Department of Biomedical & Clinical Technology, Durban University of Technology, Durban-4000, South Africa
Received: May 25, 2018; Published: May 29, 2018
Corresponding author: Dr Krishna SBN, Department of Biomedical & Clinical Technology, Durban University of Technology, Durban-4000, South Africa
DOI: 10.32474/IGWHC.2018.02.000130
Abstract
There is growing interest in health promoting benefits of probiotics as bio therapeutic agents. Probioticsare used to treat recurrent urinary tract infections, diabetes, diarrhoea, vulvovaginal candidiasis and bacterial vaginosis in women. Probiotics exert their positive effects through various mechanisms, including lowering intestinal pH, decreasing colonization and invasion by pathogenic organisms, and modifying the host immune response. There is no agreement about the minimum number of microorganisms that must be consumed to obtain a beneficial effect; however, a probiotic should typically contain several billion microorganisms to increase the chance that adequate gut colonization will occur. This review presents mechanisms of action of probiotics and briefly examines the recent developments in use of probiotics in treating both infectious and non-infectious diseases in relation to women’s health. We conclude with suggestions for future work and possible applications probiotic research.
Keywords: Lactobacillus, Probiotic, intervention, vaginal diseases, Woman health
Introduction
The World Health Organization’s (WHO) 2001 defines probiotics as live micro-organisms that, “...when administered in adequate amounts, confer a health benefit on the host [1]”.The term came into more common use after 1980. Human probiotics market size is anticipated to surpass USD 5 billion by 2024 [2] owing to its application outlook in foods & beverages, dairy & non-dairy products and fermented meat products to enhance the immunity system and improve the digestive health. These products help in curing immune response, pathogen inhibition, urogenital infections and digestive disorders in adults and nosocomial infections in infants. The outline of the concept (but not the term) is generally attributed to Nobel laureate Élie Metchnikoff, who postulated that yogurt-consuming Bulgarian peasants lived longer lives because of this custom [3]. Medical conditions that have been reportedly treated or have the potential to be treated with probiotics include diarrhoea, gastroenteritis, irritable bowel syndrome, and inflammatory bowel disease (Crohn’s disease and ulcerative colitis), cancer, depressed immune function, inadequate lactase digestion, infant allergies, failure-to-thrive, hyperlipidemia, hepatic diseases, Helicobacter pylori infections, genitourinary tract infections, and others [4,5]. Today, we live in an era of potentially tragic microbiological resistance to antibiotics [6]. With our progressively greater understanding of the influence of probiotics upon inflammation and the immune system, we have entered an era where an ideal climate prevails for the clinical extrapolation of in vitro experimentation to in vivo trials and treatments [7]. This review will endeavour to focus the use of probiotics in both infectious and non-infectious diseases in relation to women’s health. The content of this review was obtained using the search terms probiotic(s), pregnancy, women’s health and gynaecology within the Mendeley, PubMed, MEDLINE, EMBASE and Google Scholar data base.
Mechanisms of Action of Probiotics
The detailed mechanisms influencing the crosstalk between the microbe and the host are not completely understood, but there is growing evidence to suggest that the functioning of the immune system at both a systemic and a mucosal level can be modulated by bacteria in the gut [8]. In gut mucosa, pathogenic bacteria induce an inflammatory response, whilst commensal bacteria cohabit without inducing an inflammatory response [9]. A balancing act needs to be achieved between over and under stimulation of the inflammatory response. Interestingly, one of the probiotic bactericidal mechanisms via high hydrogen peroxide levels is also self-inhibitory upon the growth of lactobacilli. Orally administered strains of lactic acid bacteria (LAB) increased the number of immunoglobulin A (IgA)-producing cells in the small intestine without a concomitant increase in the CD4+ T-cell population, indicating that some LAB strains induce clonal expansion only of B cells triggered to produce IgA [10]. In a separate study [11], the cytokines released by primary cultures of intestinal epithelial cells (IEC) in animals fed with Lactobacillus casei CRL 431 or Lactobacillus helveticus R389concluded that the small intestine is the place where a major distinction would occur between probiotic LAB and pathogens. This distinction comprises the type of cytokines released and the magnitude of the response, cutting across the line that separates IL-6 necessary for B-cell differentiation, which was the case with probiotic lactobacilli, from inflammatory levels of IL-6 for pathogens.
According to the hygiene hypothesis, the increasing incidence of allergy in Westernized societies over the last decades may to some extent be explained by a reduced microbial load early in infancy [12] resulting in too little Th1 cell activity and therefore an insufficient level of IFN-γ to cross-regulate optimally Th2 cell responses. On opposing, according to the view of Classical Hygiene hypothesis, traditional commensals (lactobacilli) and other transient but harmless organisms (including saprophytic mycobacteria and helmints) induce maturation of dendritic cells(DCs) that induce Treg specific for allergens, commensals and self-antigens, that are target antigens in three groups of chronic disorders [13]. Nevertheless, probiotics do not have to be alive and whole to exert influences upon the host. Fragments of bacterial DNA, oligonucleotides, are capable of eliciting a host immune reaction [14] dead, whole or fragmented bacteria act through the same presumed mechanisms as that of live bacteria. This latter effect is at a much lower level than that seen for live bacteria [15].
Probiotics in Treatment of Bacterial Vaginosis
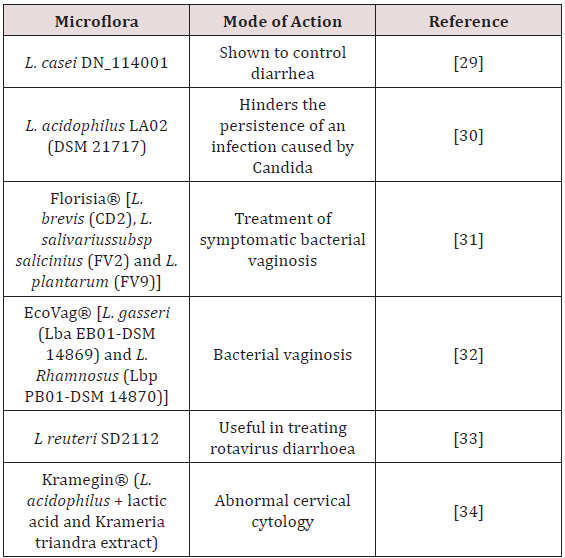
Bacterial vaginosis (BV) is the most common cause of vaginal infection in women of childbearing age, characterised by imbalance of the vaginal microbiota with a notable reduction of lactobacilli species, an overgrowth of a mixture of mostly endogenous obligate anaerobic bacteria spp. and elevated pH level in the vagina [16]. There seems to be an association between the absence of, or low concentrations of, vaginal lactobacilli and the development of BV. Many studies have suggested that the presence of H2O2-producing vaginal lactobacilli may protect against BV, although some studies do not support this hypothesis [17]. Clinical trials showed that intra-vaginal administration of Lactobacillus acidophilus for 6–12 days, or oral administration of L. acidophilus or Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14 for 2 months, resulted in the cure of BV (defined as a 0–1 positive score according to Amsel’s criteria), and/or reduced the recurrences of BV, and/ or caused an increase in vaginal lactobacilli and restoration of a normal vaginal microbiota, significantly more frequently than did a placebo, acetic acid or no treatment. Though, some trials have found no significant difference in the cure rate of BV and in the number of vaginal lactobacilli after intra-vaginal instillation of lactobacilli when compared with the effect of a placebo or oestrogen. Therefore, although the available results concerning the effectiveness of the administration of lactobacilli for the treatment of BV are mostly positive (Table 1), it cannot yet be concluded definitively that probiotics are useful for this purpose. Further, in a study [18] to compare the efficacy of combined probiotic and antibiotic therapy with antibiotic therapy alone in treatment of bacterial vaginosis, combined therapy is significantly more effective when compared with antibiotic therapy alone for the treatment of bacterial vaginosis. However, some other studies on larger sample size are required to validate these findings
Table 1: Clinical studies of Probiotics showing beneficial effects in treatment of vaginal disorders [28].
Probiotics in Treatment of Recurrent Urinary Tract Infection
Recurrent urinary tract infections (UTI) are common among young healthy women even though they generally have anatomically and physiologically normal urinary tracts. Women with recurrent UTI have an increased susceptibility to vaginal colonization with uropathogens, which is due to a greater propensity for uropathogenic coliforms to adhere to uroepithelial cells [19]. Common risk factors for recurrent UTI include sexual intercourse, use of spermicidal products, having a first UTI at an early age, and having a maternal history of UTIs [20]. Since lactobacilli dominate the urogenital flora of healthy premenopausal women, the use of probiotics, especially lactobacilli, has been considered for the prevention of UTIs. Recently, in a double-blind study [21], phase 1 trial of the safety and tolerance of Lactin-V in women with rUTI, shown that L. crispatus CTV-05 can be given as a vaginal suppository with minimal adverse effects to healthy women with a history of rUTI. Lactin-V (Osel) contains a carefully selected H2O2+ Lactobacillus crispatus strain CTV-05 isolated from a healthy woman’s vagina and was developed as a vaginal probiotic for use in patho physiological states characterized by detrimental alterations in vaginal flora, such as bacterial vaginosis (BV) [22], rUTI and potentially others. A prospective study by Yang Bob and Foley [23] suggested that bacterial vaccine Uromune® is safe and effective at preventing UTIs in women. Further research is required in larger groups of patients for longer treatment times.
Adverse Effects of Probiotics
Probiotics are mostly considered to be safe. However, some species of microorganisms that are used as probiotics have recently been isolated from infection sites, causing some concerns regarding the safety of these products [24]. Bacteremia caused by lactobacilli is rare, and data on its clinical significance is based only on case reports or data in abstract form [25]. Immuno compromised patients generally are more vulnerable to infection with pathogens and have a higher incidence of opportunistic infections [26]. However, there is no published evidence that consumption of probiotics that contain lactobacilli or bifidobacteria increases the risk of opportunistic infection among such individuals. In addition, 2 clinical studies have been conducted to assess the safety of probiotics in small groups of specific immune compromised patients (e.g., patients with HIV infection), and the findings of these studies support the safety of probiotics consumed by such groups [27-34].
Conclusion
Several in vitro and in vivo studies support the beneficial effect of some strains of lactobacilli on the restoration of the vaginal flora and the prevention of recurrent UTIs. Probiotics use do not represent a complete cure, but evidence is accumulating that the use of proven probiotic strains and manipulation of the host’s own intestinal and vaginal/urethral microbiota will provide valuable opportunities to help restore and maintain urogenital health. Once appropriate product formulations with supporting clinical data become available, it will be up to the physician to determine their place in patient management.
Conflict of Interest
Author declares no conflict of interest.
References
- Hill C, Francisco Guarner, Gregor Reid, Glenn R Gibson, Daniel J Merenstein, et al. (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology &Amp Hepatology 11: pp. 506-514.
- (2018) Probiotics Market Size By Ingredients.
- Brown AC, A Valiere (2004) Probiotics and medical nutrition therapy. Nutr Clin Care 7(2): 56-68.
- Adam JK, O Bharti, SBN Krishna (2012) Probiotics: Recent Understandings and Bio-medical Applications. Current Trends in Biotechnology and Pharmacy 6(1): 1-14.
- Fleet M, PK Rahman (2017) Probiotics and Their Health Benefits. Microbial Functional Foods and Nutraceuticals pp. 267-279.
- Griffin C (2015) Probiotics in obstetrics and gynaecology. Australian and New Zealand Journal of Obstetrics and Gynaecology. 55(3): 201-209.
- Lindsay KL, Brennan L, Kennelly MA, Maguire OC, Smith T, et al. (2015) Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. American Journal of Obstetrics & Gynecology 212(4): 496. e1-11.
- Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of Action of Probiotics: Recent Advances. Inflammatory Bowel Diseases 15(2): 300-310.
- Tomás MSJ, E Bru, ME Nader-Macías (2003) Comparison of the growth and hydrogen peroxide production by vaginal probiotic lactobacilli under different culture conditions. American Journal of Obstetrics & Gynecology 188(1): 35-44.
- Galdeano CM, G Perdigon (2006) The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clinical and Vaccine Immunology 13(2): 219-226.
- Vinderola G, C Matar, G Perdigon (2005) Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: involvement of toll-like receptors. Clinical and diagnostic laboratory immunology 12(9): 1075-1084.
- Kiseleva E, G Novik (2013) Probiotics as immunemodulators: substances, mechanisms and therapeutic benefits.
- Mohamadzadeh M (2010) Induction of protective immunity against microbial challenge by targeting antigens expressed by probiotic bacteria to mucosal dendritic cells. Current HIV research 8(4): 323-329.
- Shimosato T, H Kitazawa (2013) Immunogenics: immunostimulatory oligodeoxynucleotides from probiotics. Probiotics: Immunobiotics and Immunogenics, CRC Press, Boca Raton pp. 336-350.
- Rodriguez AV, et al. (2013) Immunogenics: extracellular bacterial compounds as mediators of lactic acid bacteria-target cell interaction. Probiotics: Immunobiotics and Immunogenics pp. 354.
- Krishna S, SL Wilson, JK Adam (2017) The Vaginal Microbiota in Women Health and Disease: Current Understanding and Future Perspectives-A Review. Current Trends in Biotechnology & Pharmacy 11(2): 190-205.
- Falagas ME, GI Betsi, S Athanasiou (2007) Probiotics for the treatment of women with bacterial vaginosis. Clinical Microbiology and Infection 13(7): 657-664.
- Arooj A, Naheed Bano, Rukhsana Nazir, Rizwana Chaudhri (2017) Comparison of Combined Probiotic and Antibiotic Therapy Versus Antibiotic Therapy Alone in Treatment of Bacterial Vaginosis. Journal of the Society of Obstetrics and Gynaecologists of Pakistan 2(2).
- Hooton TM (2001) Recurrent urinary tract infection in women. International Journal of Antimicrobial Agents 17(4): 259-268.
- Foxman B (1990) Recurring urinary tract infection: incidence and risk factors. American journal of public health 80(3): 331-333.
- Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, et al. (2011) Randomized, Placebo-Controlled Phase 2 Trial of a Lactobacillus crispatus Probiotic Given Intravaginally for Prevention of Recurrent Urinary Tract Infection. Clinical Infectious Diseases 52(10): 1212-1217
- Hemmerling A, Harrison W, Schroeder A, Park J, Korn A, et al. (2009) Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV- 05 (LACTIN-V) for the prevention of bacterial vaginosis. Sexually transmitted diseases 36(9): 564-569.
- Bob Y, F Stephen (2018) First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU International 121(2): 289-292.
- Falagas ME, Harrison W, Schroeder A, Park J, Korn A (2006) Probiotics for Prevention of Recurrent Urinary Tract Infections in Women. Drugs 66(9): 1253-1261.
- Husni RN, Gordon SM, Washington JA, Longworth DL (1997) Lactobacillus bacteremia and endocarditis: review of 45 cases. Clinical Infectious Diseases 25(5): 1048-1055.
- Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, et al. (2003) Safety of Probiotics That Contain Lactobacilli or Bifidobacteria. Clinical Infectious Diseases 36(6): 775-780.
- Cunningham-Rundles S, Ahrné S, Bengmark S, Johann-Liang R, Marshall F, et al. (2000) Probiotics and immune response. The American journal of gastroenterology 95(1): S22-S25.
- Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T (2016) Mechanisms and therapeutic effectiveness of lactobacilli. Journal of Clinical Pathology 69(3): 187-203.
- Dietrich CG, T Kottmann, M Alavi (2014) Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduces antibiotic-associated diarrhoea. World Journal of Gastroenterology 20(42): 15837-15844.
- Murina F, Graziottin A, Vicariotto F, De Seta F (2014) Can Lactobacillus fermentum LF10 and Lactobacillus acidophilus LA02 in a slow-release vaginal product be useful for prevention of recurrent vulvovaginal candidiasis?: A clinical study. J Clin Gastroenterol 48(1): S102-S105.
- Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, et al. (2009) Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 15(1): 67-74.
- Larsson PG, Stray-Pedersen B, Ryttig KR, Larsen S (2008) Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 8: p. 3.
- Cadieux P, et al. (2008) Evaluation of reuterin production in urogenital probiotic Lactobacillus reuteri RC-14. Applied and environmental microbiology 74(15): 4645-4649.
- Di Pierro F, G Di Paola, P Risso (2014) Role of a medical device for intravaginal use in improving the quality of the colposcopic examination and the anatomical/pathological reading of the cytological test and biopsy. Acta Biomed 85(2): 121-126.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...















