Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Review Article(ISSN: 2637-4544) 
Ovarian Mucinous Borderline Tumor with Malignant Muralnodules: A Report of Two Cases Volume 4 - Issue 2
Graham A*, Kazerouni H and Sur M
- Department of Pathology and Molecular Medicine, Juravinski Hospital, Hamilton, Ontario
Received: November 25, 2019; Published: January 21, 2020
Corresponding author: Graham A, Department of Pathology and Molecular Medicine, Juravinski Hospital, Hamilton, Ontario
DOI: 10.32474/IGWHC.2020.04.000181
Keywords: Mucinous ovarian tumor; Mural nodule; Anaplastic carcinoma; Angiosarcoma; Poorly differentiated carcinoma; Poor prognosis
Introduction
Mucinous tumors of the ovary account for about 15% of all ovarian tumors; 80% are benign, while only 3-4% are invasive carcinoma; the remainder are borderline tumors [1]. Rarely, mucinous tumors may exhibit mural nodules containing a carcinomatous or sarcomatous component; these mural nodules are more commonly associated with mucinous borderline tumors but can also arise within mucinous carcinoma [1, 2]. Prat and Scully first described mucinous ovarian tumors with mural nodules in a series of 7 cases in 1979; these lesions were termed mucinous tumors with sarcoma-like nodules [3,4]. However, classification of mural nodules arising in mucinous ovarian tumors remains unclear, and several authors have proposed different classification systems [5-14]. Anaplastic carcinoma is the most common type of mural nodular malignancy; these tumors can infiltrate borders and invade blood vessels; they readily metastasize and generally have a poor prognosis [6,7]. We describe two cases of postmenopausal women with malignant mural nodules arising from mucinous borderline tumors of the ovary.
Case reports
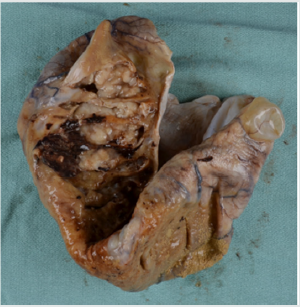
Figure 1: A Photograph showing ovarian mass containing mural nodules, B: Mural nodules observed in wall of ovarian mass.
Case 1: A 58-year-old female presented with a several month history of weight gain and abdominal discomfort; CT scan of the abdomen and pelvis demonstrated a large pelvic mass arising from the uterus and right adnexa, with septations and enhancing components. Preoperative tumor markers were elevated: CA-125 at 367, and CA19-9 at 2266. Peritoneal ascitic fluid contained atypical cells suspicious for malignancy. Hysterectomy, bilateral salpingooophorectomy, and omentectomy were performed. Grossly, the ovarian lesion was 31.0cm x 29.0 cm x 15.0cm in size. Capsule was collapsed, and mucoid material was observed oozing from the surface, which was otherwise whitish and smooth; cut surfaces revealed multiloculation, with clear mucinous material (Figure 1A). Several solid areas consisting of firm, tan-white, somewhat mucoid tissue were noted in the wall of the ovarian mass (Figure 1B). Histology revealed a complex mucinous neoplasm with benign, borderline, and malignant change, containing multiple solid nodules of anaplastic carcinoma (Figure 2).
Figure 2: Microscopic appearance of ovarian tumor (hematoxylin & eosin staining). A. Mucinous borderline tumor, B. Mucinous borderline tumor with mural nodule containing focus of anaplastic carcinoma, admixed with discrete borders.
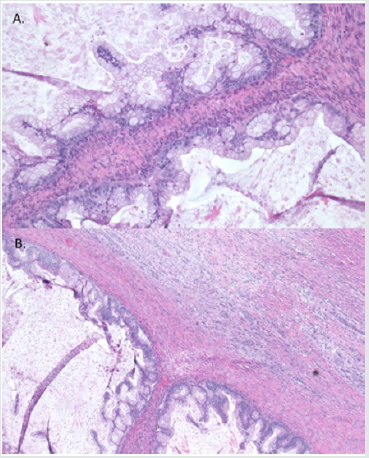
Figure 3: Microscopic appearance of mural nodule containing anaplastic carcinoma (hematoxylin & eosin staining). Tumor cells showed eosinophilic cytoplasm, high grade eccentric nuclei, and prominent nucleoli.
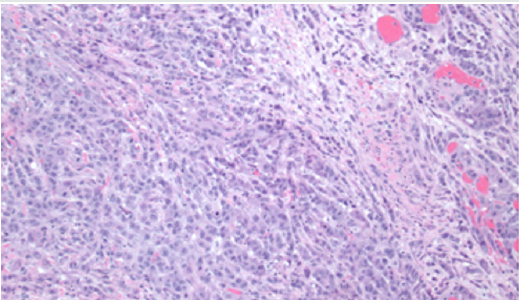
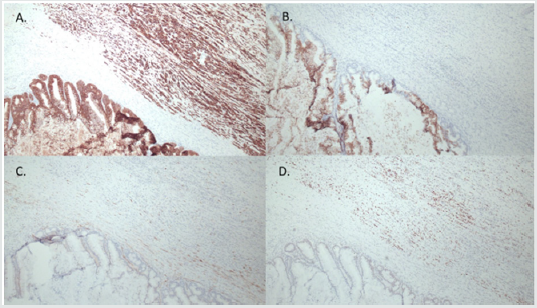
A small focus of intraepithelial carcinoma was identified within the mucinous borderline tumor. Tumor cells had eosinophilic cytoplasm, high grade eccentric nuclei, and prominent nucleoli; some areas showed spindle cell proliferation and rhabdoid differentiation (Figure 3). The ovarian surface was involved by anaplastic carcinoma; this component was also present in lymphovascular spaces in the left fallopian tube, paratubal soft tissue, and paradnexal tissue. A macroscopic extraperitoneal focus of anaplastic carcinoma was detected in the omentum. The anaplastic carcinoma component showed diffuse immunopositivity for CK7, and weak positivity for CK20; p53 was diffusely positive (Figure 4). The mucinous borderline component was also positive for CK7 and CK20, as well as PAX8, CDX2, and villin. In both components, p16 showed mosaic staining, and ER and PR were negative.
Figure 4: Immunohistochemical features of ovarian lesion, showing mucinous borderline tumor and mural nodule with anaplastic carcinoma. Both the mucinous borderline and anaplastic carcinoma components showed diffuse immunopositivity for CK7 (A), the anaplastic component showed weak positivity for CK20, while the borderline component was strongly positive for CK20 (B); both components showed mosaic staining for p16 (C), anaplastic component was diffusely positive for p53 (D).
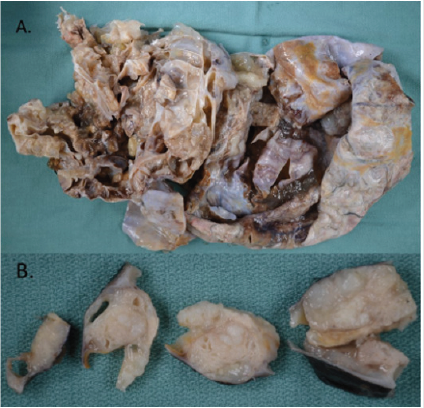
Case 2: A 52-year-old female presented with a two-month history of abdominal discomfort, bloating, and weight gain. CT scan of the abdomen and pelvis showed a large cystic mass with internal septations and several solid components, favoured to arise from the right ovary. Preoperative CA-125 was slightly elevated at 42. Peritoneal ascitic fluid was negative for malignant cells. Hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendectomy, and peritoneal biopsies were performed. Grossly, the ovarian lesion was 30.0 cm x 18.0 cm x 15.0 cm in size with a smooth, intact capsule, and one 40 mm solid nodule, which consisted of tan, hemorrhagic, friable, multicystic tissue (Figure 5). Microscopically, a mucinous borderline tumor with intraepithelial carcinoma and a mural nodule was observed. The nodule was predominantly composed of pleomorphic spindle cells, but also had an epithelial component (Figure 6). Spindle cells contained hyperchromatic nuclei and were arranged in a vaguely fascicular pattern with some vascular-like spaces; no vascular invasion was seen (Figure 6).
Figure 6: Microscopic appearance of ovarian tumor (hematoxylin & eosin staining). A. Mucinous borderline tumor. B. Mucinous borderline tumor with mural nodule containing pleomorphic spindle cells. C. Mural nodule containing pleomorphic spindle cells and poorly differentiated carcinoma. D. Angiosarcoma component.
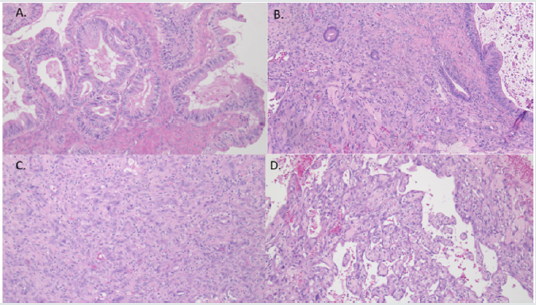
Figure 7: Immunohistochemical features of ovarian lesion, showing a small focus of mucinous borderline tumor and mural nodule with poorly differentiated carcinoma. Both the mucinous borderline and poorly differentiated carcinoma components showed diffuse immunopositivity for CKAE1/AE3 (A), and CAM5.2 (B).
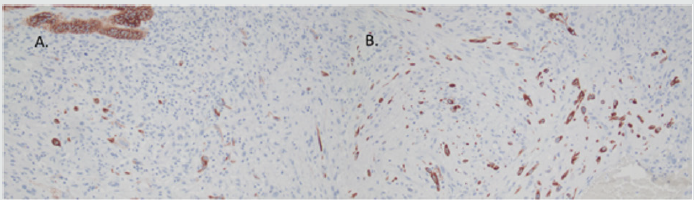
Figure 8: Immunohistochemical features of ovarian lesion, showing mural nodule with spindle cell component consistent with angiosarcoma, which showed diffuse immunopositivity for vimentin (A), CD31 (B and C), and ERG (D).
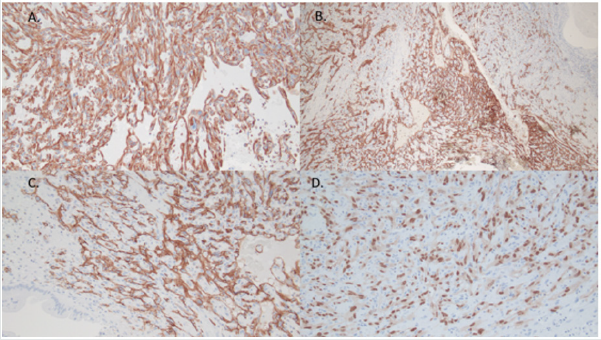
Immunohistochemical stains for CK AE1/AE3 and CAM5.2 highlighted the mural nodule’s epithelial component, which was consistent with invasive poorly differentiated carcinoma (Figure 7). The spindle cell component was negative for pancytokeratins, and diffusely positive for vimentin, CD31 and ERG; consistent with angiosarcoma (Figure 8). The case was sent for outside consultation, and the mural nodule was determined to be a malignant spindle cell neoplasm consistent with angiosarcoma with admixed foci of poorly differentiated carcinoma. Ultimately, the mural nodule was classified as a malignant mesodermal mixed tumor (carcinosarcoma).
Discussion
Mucinous borderline tumors of the ovary containing malignant
mural nodules are rare entities; a small number of cases have been
described since 1979 [6,8,9,14]. Prat and Scully (1979) proposed
a classification system dividing mural nodules into three classes
based on histology: type 1 nodules (pleomorphic and epulis
like), type 2 nodules (pleomorphic and spindle cell) and type 3
nodules (giant cell-histiocytic) [3]. Other reports have described
mural nodules based on gross examination: sarcoma-like, which
are typically multifocal and contain large areas of hemorrhage,
anaplastic or undifferentiated carcinomas, which are often
multifocal but less hemorrhagic than sarcoma-like mural nodules;
and sarcoma, which tend to be solitary nodules [10-12]. Additional
reports have classified mural nodules into five or six groups:
anaplastic carcinoma, sarcoma, sarcoma-like, mixed, and smooth
muscle sarcoma; carcinosarcoma may be considered a grouping, as
well as leiomyoma [1,5,14,15].
In our two cases, the patients were close in age and presented
with similar symptoms: weight gain and abdominal discomfort.
Imaging of both ovarian lesions showed similar large cystic pelvic
masses with septations. Gross examination of the lesions also
revealed similarities: both lesions were over 30cm in greatest
dimension, multiloculated, and contained solid nodules within
the wall. Microscopic features of the mural nodule in case 1 were
easy to discern, given the eosinophilic cytoplasm, high grade
eccentric nuclei, with focal spindle cell proliferation, which are
characteristic of anaplastic carcinoma. The mural nodule in case
2, however, had both a spindle cell and epithelial component and
required expert consultation to diagnose as angiosarcoma with
poorly differentiated carcinoma. Therefore, the mural nodule in
case 2 may be categorized as carcinosarcoma, mixed, or sarcoma,
depending on which classification scheme is referred to. Prognosis
for patients with mural nodules arising from mucinous borderline
tumors of the ovary is generally thought to be poor, especially for
sarcomatous and anaplastic mural nodules [7-13].
A more favorable prognosis is expected in patients where the
malignant mural nodule is confined to the ovary and/or lacks
vascular invasion [9-15]. In addition, type 3 nodules (giant cellhistiocytic)
in the classification proposed by Prat and Scully (1979)
are also thought to behave in a benign manner and therefore portend
a good prognosis [3]. However, a larger study of 34 cases of anaplastic
carcinoma arising in mucinous ovarian tumors concluded that foci
of anaplastic carcinoma in unruptured stage I mucinous tumors
may not foretell an unfavorable prognosis; one third of patients
who were followed up died within 8 months, but these were stage
>1C tumors; only one patient with stage 1A disease died [14]. In
case 2, mural nodules had not disseminated beyond the ovary, and
no lymphovascular invasion was identified. The patient remains
free of local recurrence or metastases approximately 3 years after
surgery; she continues to undergo active surveillance every 6
months with CT scans. The patient in case 1 is only a few months
post operative and is currently disease free despite the presence
of lymphovascular invasion and dissemination of the anaplastic
component to the ipsilateral fallopian tube, paratubal soft tissue,
paradnexal tissue, and omentum (pT3c). She is currently receiving
chemotherapy with Carboplatin and Paclitaxol. A Lynch Study
demonstrated intact staining for both the mucinous borderline
tumor and anaplastic carcinoma component in this lesion.
Conclusion
We present two cases of a rare entity: mural nodules arising in a mucinous borderline tumor of the ovary. In case 1, anaplastic carcinoma arose in a nodule, and metastases of this primary neoplasm were observed diffusely around the pelvis; precluding the fact that anaplastic carcinoma within a mucinous neoplasm leads to aggressive behaviour. Case 2 was unusual since two different malignant components were observed within the mural nodule. There is conflicting evidence in the literature regarding progression of mural nodules; our first case was limited to the ovary, and the patient experienced a favourable outcome; the second case was an anaplastic mural nodule that disseminated around the pelvis, and the patient is still undergoing treatment; these results seem to fit with past reports detailing behaviour of these tumors. Ultimately, close follow up of patients with mural nodules arising in mucinous borderline tumors of the ovary is imperative given the tendency of these lesions to spread to lymphovascular spaces and metastasize. In addition, progression of malignant neoplasms arising in mucinous borderline tumors should be included in the differential diagnosis when undifferentiated tumors are identified in the omentum and the patient has a history of a pelvic mass.
References
- Clement PB, Young RH (2014) Atlas of Gynecologic Surgical Pathology.
- Hong JH, Park S, Kim TJ (2019) Anaplastic ovarian carcinoma arising from a background of mucinous carcinoma with fatal outcome: case report and literature review. Annals of Clinical Case Reports 4: 1-3.
- Prat J, RE Scully (2013) Ovarian mucinous tumors with sarcoma-like mural nodules: a report of seven cases. Cancer Int J Clin Exp Pathol 6(8): 1688-1692.
- Akita H, Kushima M, Okubo, Takashi Okia T, H Ota (2005) A case of ovarian mucinous cystic tumor with mural nodules including a few small foci of carcinomatous lesion. Showa Univ J Med Sci 17: 63-69.
- Selvaggi SM (2000) Tumors of the Ovary, Maldeveloped Gonads, Fallopian Tube, and Broad Ligament. Arch Pathol Lab Med 124(3): 477.
- Yang S, Wang L, K Sun (2019) Ovarian mucinous cystic tumor associated with sarcomatous mural nodule and benign Brenner tumor: A case report and literature review. Medicine (Baltimore) 98(3): e14066.
- Baergen RN, JL Rutgers (1994). Mural nodules in common epithelial tumors of the ovary. Int J Gynecol Pathol 13(1): 62-72.
- Okamura T, Muronosono E, Tsubuku M, Terao Y, Takeda S, et al. (2018) Anaplastic carcinoma in ovarian seromucinous cystic tumor of borderline malignancy. J ovarian Res11: 77.
- Chakrabarti S, Konar A, Biswas S, S Das (2005) Sarcoma-like mural nodules in ovarian mucinous cystadenomas-A report of 2 cases. Indian J Med Sci 59: 499-502.
- McFarland M, Dina R, Fisher C, McCluggage WG (2015) Osteosarcoma as malignant mural nodule in ovarian mucinous neoplasms of intestinal type: report of 2 cases. Int J Gynecol Pathol 34(4): 369-373.
- Desouki MM, Fadare O, Kanbour A, Kanbour-Shakir A (2014) Immunophenotype and K-RAS mutation in mucinous ovarian adenocarcinoma with mural nodule of high grade sarcoma: case report. Int J Gynecol Pathol 33(2): 186-190.
- Zhang Y, Yuan Z, Sun K (2017) Ultrasonic and pathological characteristics of ovarian mucinous cystic tumors with malignant mural nodules. Medicine (Baltimore) 96(45): e8636.
- Mhawech-Fauceglia P, Ramzan A, Walia S, Pham HQ, Yessaian A (2016) Microfocus of anaplastic carcinoma arising in mural nodule of ovarian mucinous borderline tumor with very rapid and fatal outcome. Int J Gynecol Pathol 35(4): 348-351.
- Provenza C, Young RH, J Prat (2008) Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathological study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcomalike mural nodules. Am J Surg Pathol 32(3): 383-389.
- Suurmeijer AJ (1991) Carcinosarcoma-like mural nodule in an ovarian mucinous tumour. Histopathology 18(3): 268-271.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...