Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Research Article(ISSN: 2637-4544) 
Maternal Hematological Aspects in Correlation to Placental and Pathological Findings in Gestations with Growth Restrictive Issues Volume 3 - Issue 3
Walid M Elnagar1*, Maha Abdelhamid Amin2, Mai M Abdelwahab3 and Samia Hussein4
- 1 Department of Obstetrics and Gynecology, Zagazig University, Egypt
- 2 Department of Physiology, Zagazig University, Egypt
- 3 Department of Pathology, Zagazig University, Egypt
- 4 Department of Medical Biochemistry and Molecular Biology, Zagazig University, Egypt
Received:July 09, 2019; Published: July 15, 2019
Corresponding author: Wael S Nossair, Department of Obstetrics and Gynecology, Zagazig University, Egypt
DOI: 10.32474/IGWHC.2019.03.000164
Abstract
Background: The placental circulation and microvasculature is a highly sensitive system particularly during development. A growing research interest all over the globe is to utilize the biomarkers in diagnosis of common clinical case scenario to enhance the level of medical care.
Aim: To assess and evaluate the placental and umbilical cord histopathological findings in cases of intrauterine growth restriction and their correlation to second gestational trimester maternal hematological indices.
Methodology: A prospective case-control clinical research trial conducted study between January 2016 and December 2018 at Zagazig university. Hospital research data have been gathered from the medical records; blood indices have been obtained within second gestational trimester. Study subjects have been categorized as IUGR when the expected fetal weight on the 20th gestational week of gestation was under the 10th centile as corresponding gender, and gestational age.
Results: Umbilical cord and placental pathological findings among both research groups in which there was no statistical significant difference between IUGR and control research groups as regards Vena umbilicalis vessel wall thickness, Vena umbilical is lumen area, arteria umbilicalis vessel wall thickness, ratio of star-shaped arteries, umbilical cord diameter, longest placental diameter, placental thickness (p values = 0.130, 0.672, 0.398, 0.061, 0.221, 0.087, 0.276, 0.269 consecutively) however arteria umbilicalis lumen area, number of placental pathologies present, native placental volume, placental volume after formalin fixation, placental weight after formalin fixation, shortest placental diameter, placental coefficient are statistically significant difference (p values<0.001).
Conclusion: The current study elucidates the importance of histopathological evaluation of the placentas of growth restricted fetuses in order to understand the histopathological development of this disease in an effort to innovatively develop an effective management line for this common obstetric clinical case scenario.
Keywords: Hematological aspects, Histopathological findings, Intrauterine growth restriction
Introduction
Intra uterine growth restriction (IUGR) is a common clinical scenario that is presented in every day obstetric practice raising morbidity and mortality issues at both maternal and neonatal levels particularly in the presence of comorbid medical diseases with pregnancies [1-3]. The placental circulation and microvasculature are a highly sensitive system particularly during development. A growing research interest all over the globe is to utilize the biomarkers in diagnosis of common clinical case scenarios to enhance the level of medical care, however the issues of sensitivity and sensitivity of various maternal biomarkers is a great area of research efforts. Maternal hematological changes during pregnancy could have a great impact on the placental development and maturity [4-7].
Researchers are gaining interest to reveal and display any correlation between placental histopathological finding between maternal hematological indices in various obstetric clinical scenarios such as chorioamnionitis condition, however as complex issue of intrauterine growth restriction still there is requirements to put more investigative efforts to elucidate the value and linkage between maternal hematological indices and placental histopathology issues in cases with IUGR [8-11].
An interesting fact to mention is that the placental size, weight, and shape have a wide range of normal values besides placental size and integrity at cellular, physiological levels is crucial for fetal nutritive and respiratory functions. Furthermore, the placenta is a complex organ that have endocrinal and enzymatic activity that could be easily affected by maternal hematological issues [12-15]. Researchers in prior research studies have revealed that placentas of neonates affected by intrauterine restrictive issues are shown to be smaller in diameters and reduced in overall weight and volume in comparison to normal-weight neonates [16,17]. Furthermore, placental vascular pathological issues such as calcification, decreased cyto trophoblastic proliferation and perivillous fibrin deposits are correlated and linked in previous research studies to intrauterine growth restrictive issues whether symmetrical or asymmetrical [18,19].
Aim of the work
To assess and evaluate the placental and umbilical cord histopathological findings in cases of intrauterine growth restriction and their correlation to second gestational trimester maternal hematological indices.
Methodology
A prospective case-control clinical research trial conducted study between January 2016 and December 2018 at Zagazig university hospital research data have been gathered from the medical records, blood indices have been obtained within second gestational trimester study subjects have been categorized as IUGR (10 cases) when the expected fetal weight on the 20th gestational week of gestation was under the 10th centile as corresponding gender and gestational age.
Exclusive research criteria were as follows cases having twin gestations, preterm deliveries, congenital anomalies, hypertension with pregnancy, DM whether gestational or pregestational smokers and cases with history of substance abuse cases recruited for the research study didn’t have immunological, cardiovascular, gastrointestinal, or pulmonary illnesses.
Sonographic assessment has been conducted by the same sonographer to prevent interobserver variability issues. Gestational age has been calculated according to the first day of the last menstrual period and sonographic biometry indices (CRL and BPD) between the 9th and 11th gestational weeks. At the 20th-24th gestational weeks, obtained fetal biometric indices were implemented to evaluate expected fetal weight using Hadlock formulas after delivery, the neonatal weight and placental volume have been measured.
Umbilical cord and placental sample assessment after around 3-7 days of fixation by formalin, placental weight and volume had been measured with histopathological gross examination and the umbilical cord was executed by an experienced pathologist. The obtained placenta has been cut along the longest diameter in 1cm thick strips and the thickness of the tissue was measured at the site of umbilical cord insertion. Umbilical cord samples were taken from the placental end and divided into transverse slices measuring 4mm, in a perpendicular manner to the umbilical cord. Histological slices have been dehydrated in graded ethanol series, cleaned in xylene, and embedded in paraffin. After hematoxylin/eosin (HE) staining process, histological samples have been examined microscopically.
Statistical analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when parametric and median with inter-quartile range (IQR) when nonparametric. Also, qualitative variables were presented as number and percentages. The comparison between groups regarding qualitative data was done by using Chi-square test and/or Fisher exact test when the expected count in any cell found less than 5. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test while with nonparametric data were done by using Mann-Whitney test. Spearman correlation coefficients were used to assess the correlation between quantitative parameters. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
Results
Table 1 reveals and displays that there was no statistically significant difference between IUGR and control research groups as regards the MCV, RBC count, Hb, Hct, platelet count, MPV, prothrombin time, INR, APTT, (p values=0.123, 0.571, 0.538, 0.526, 0.132, 0.492, 0.136, 0.314, 0.281 consecutively); however concerning umbilical artery S/D ratio was statistically significantly higher among IUGR research group, on the other hand there was no statistically significant difference between IUGR and control research groups as regards maternal age and BMI, parity with p values 0.122, 0.342, 0.623, consecutively. Statistical significant difference existed concerning birth weight of neonates being higher in control research group (p value<0.001), although APGAR score at 1 min was statically significantly higher among control research group (p value=0.042); the APGAR score at 5 and 10 min wasn’t statically significantly different between both research groups (p values = 0.119, 0.321 consecutively), finally gestational age and gender among both research groups wasn’t statistically significantly different (p values =0.061,0.603 consecutively) (Table 1).
Table 2 and Figure 1 reveals and displays the placental histopathological findings among both IUGR and control research groups in which there was no statistical significant difference as regards focal calcification, amnion nodosum, villitis, hematoma (p values=0.080, 0.681, 0.681, 0.469 consecutively) whereas Villous hypo vascularization, Villous hypoplasia, Intervillous fibrin deposition, Syncytial node, Non-conversion of maternal vessels were statistically significantly more frequent among IUGR research group (p values <0.001, 0.010, 0.022, <0.001, 0.022 consecutively) (Table 1) (Figures 1-4).
Table 1: Clinical research data of the mothers and newborns.
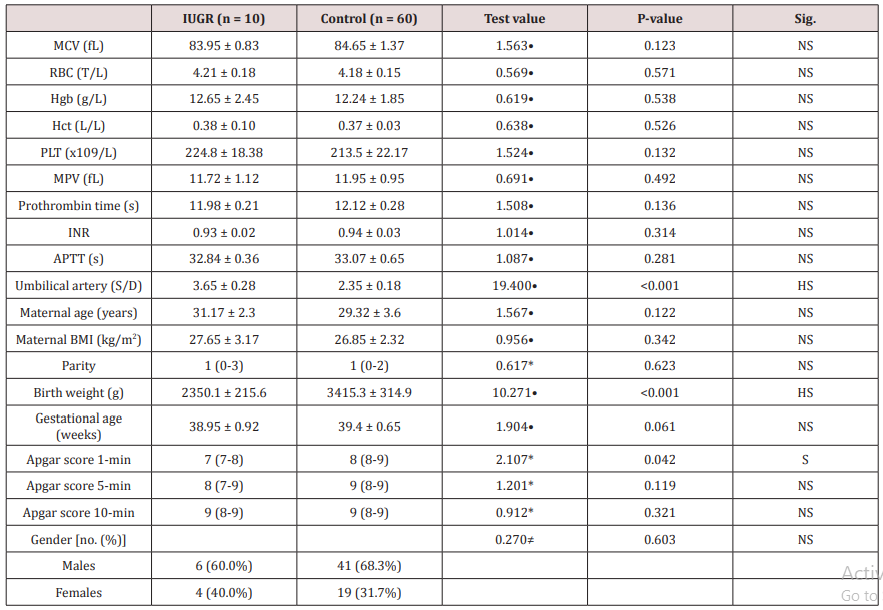
*: Data were presented as median with (IQR) and compared using Mann-Whitney test
•: Data were presented as mean with SD and compared using Independent t-test
≠: Data were presented as numbers and percentages and compared using chi-square test
Table 2: Placental histopathologic alterations.
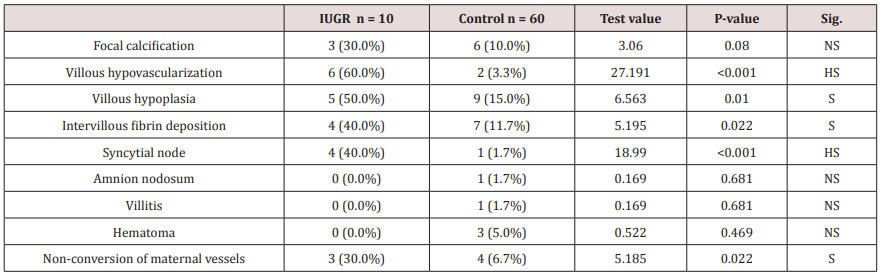
Data were presented as numbers and percentages and compared using Chi-square test and or Fisher exact test
Figure 1: Placental histopathologic findings among the two research groups (*) indicate statistically significant difference among most findings investigated.

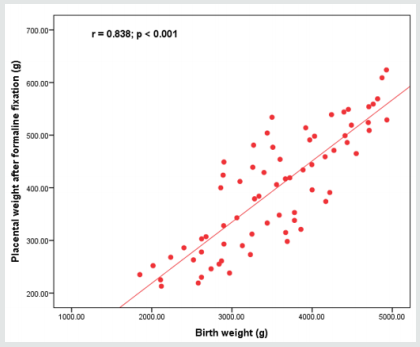
Figure 2: Placental morphometry; placental volume measured after delivery shows the strongest correlation with birth weight, not the placental volume.
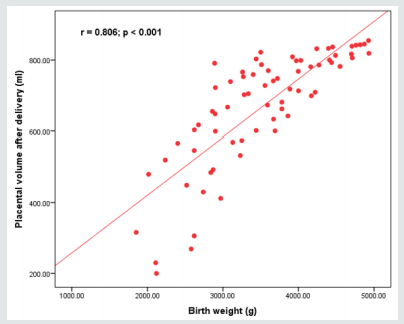
Table 3 reveals and displays umbilical cord and placental pathological findings among both research groups in which there was no statistical significant difference between IUGR and control research group as regards vena umbilicalis vessel wall thickness, vena umbilical is lumen area, Arteria umbilicalis vessel wall thickness, Ratio of star-shaped arteries, Umbilical cord diameter, Longest placental diameter, placental thickness (p values =0.130, 0.672, 0.398, 0.061, 0.221, 0.087, 0.276, 0.269 consecutively) however Arteria umbilicalis lumen area, number of placental pathologies present, Native placental volume, placental volume after formalin fixation, Placental weight after formalin fixation, Shortest placental diameter, placental coefficient (p values<0.001) (Table 3).
Table 3: Umbilical cord and placental pathological findings.
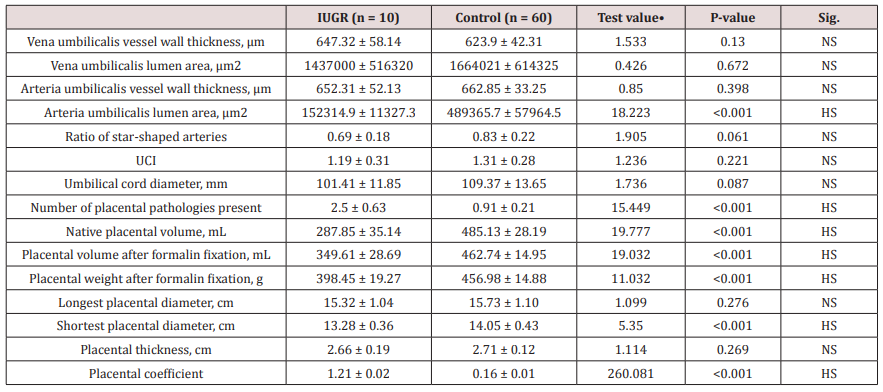
•: Data were presented as mean with SD and compared using Independent t-test
Discussion
Placental development and integrity are a cornerstone process for normal fetal growth pattern and development, however histopathological findings of placentas even in cases with no medical disorder with pregnancy reveal that intrauterine growth restriction could be associated and correlated to considerable placental abnormal histopathological findings [20,21]. Research studies similar to the current research in approach and methodology had revealed that the linkage between total placental volume and neonatal birth weight is more statistically significant than the correlation between neonatal birth weight and placental weight [22,23].
Furthermore, prior research efforts in harmony with the current research study findings the umbilical artery lumen area was statistically significantly reduced in intrauterine growth restrictive gestations. Those research findings in harmony and great similarity to the current study findings could be justified by the basic physiological fact that the placental normal vascularity is of cornerstone importance in maintenance and integrity of the fetal growth and development process and any affection of the vascular performance of the placenta is associated with reduced fetal growth potential [1,4,7,9].
Interestingly prior investigators have revealed and displayed that from the maternal peripheral venous blood indices, only the platelet count is statistically correlated to growth restrictive issues observed clinical findings [10,14,17]. Clinical scenarios of preeclampsia are associated with raised maternal mononuclear cells and elevated cytokines level that could probably cause endothelial dysfunction and consequently intrauterine growth restrictive issues [19,23].
As regards the reliable maternal blood indices that could be implemented in detectability of growth restrictive issues only the platelet count reveals a statistically significant correlation as shown in the current study findings [2,8,17]. Another prior research team of investigators have shown among their research study findings that the placental volume indices obtained after delivery have a more powerful correlation to neonatal birth weight more than placental weight indices in correlation to neonatal birth weight [4,9,15].
In distinctive manner placentas in intrauterine restrictive gestations have tendency to be more oval in shape and thicker than placentas of healthy fetuses, prior histopathological studies of fetal growth restricted gestations pregnancies have shown that calcification and reduced villous vascularity are a common characteristic finding of placentas in those pregnancies [13,15,22].
Conclusion
The current study elucidates the importance of histopathological evaluation of the placentas of growth restricted fetuses in order to understand the histopathological development of this condition in an effort to innovatively develop an effective management line for this common obstetric clinical case scenario. This is only a small pilot study and further research is needed.
References
- Kim CJ, Romero R, Chaemsaithong P, Kim JS (2015) Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. American Journal of Obstetrics and Gynecology 213: S53-S69.
- Bardien N, Whitehead CL, Tong S, Ugoni A, McDonald S, et al. (2016) Placental insufficiency in fetuses that slow in growth but are born appropriate for gestational age: A prospective longitudinal study. PLoS ONE 11: 1-13.
- Melamed N, Ryan G, Windrim R, Toi A, Kingdom J (2016) Choice of formula and accuracy of fetal weight estimation in small-for-gestational-age fetuses. Journal of Ultrasound in Medicine 35: 71-82.
- Poljak B, Agarwal U, Jackson R, Alfirevic Z, Sharp A (2017) Diagnostic accuracy of individual antenatal tools for the detection of the small for gestational age newborn. Ultrasound in Obstetrics & Gynecology 49: 493-499.
- Schwartz N, Wang E, Parry S (2012) Two-dimensional sonographic placental measurements in the prediction of small-for-gestational age infants. Ultrasound in Obstetrics & Gynecology 40: 674-679.
- Plasencia W, Akolekar R, Dagklis T, Veduta A, Nicolaides K (2011) Placental volume at 11-13 weeks’ gestation in the prediction of birth weight percentile. Fetal Diagnosis and Therapy 30: 23-28.
- Chen KH, Chen LR, Lee YH (2011) Exploring the relationship between preterm placental calcification and adverse maternal and fetal outcome. Ultrasound in Obstetrics & Gynecology 37: 328-334.
- Ohgiya Y, Nobusawa H, Seino N (2016) MR Imaging of Fetuses to Evaluate Placental Insufficiency. Magnetic Resonance in Medical Sciences 15: 212-219.
- Benirschke K, Burton GJ, Baergen RN (2012) Pathology of the Human Placenta. [6th edn] Springer.
- Higgins LE, Simcox L, Sibley CP, Heazell AE, Johnstone ED (2016) Third trimester placental volume and biometry measurement: A methoddevelopment study. Placenta 42: 51-58.
- Nkwabong E, Kamgnia Nounemi N, Sando Z, Mbu RE, Mbede J (2015) Risk factors and placental histopathological findings of term born low birth weight neonates. Placenta 36(2): 138-141.
- Salavati N, Sovio U, Mayo RP, Charnock-Jones DS, Smith GC (2016) The relationship between human placental morphometry and ultrasonic measurements of utero-placental blood flow and fetal growth. Placenta 38: 41-48.
- Javor D, Nasel C, Schweim T, Dekan S, Chalubinski K, Prayer D (2013) In vivo assessment of putative functional placental tissue volume in placental intrauterine growth restriction (IUGR) in human fetuses using diffusion tensor magnetic resonance imaging. Placenta 34(8): 676-680.
- Larsen S, Bjelland EK, Haavaldsen C, Eskild A (2016) Placental weight in pregnancies with high or low hemoglobin concentrations. Eur J Obstet Gynecol Reprod Biol 206: 48-52.
- Burke N, Flood K, Muellers S, Murray A, Dunne E, et al. (2016) Reduced spontaneous platelet aggregation: a novel risk factor for adverse pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 199: 132-136.
- Sovio U, White IR, Dacey A, Pasupathy D, Smith GC (2015) Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet 386(10008): 2089-2097.
- Azizieh FY, Raghupathy RG (2015) Tumor necrosis factor-α and pregnancy complications: a prospective study. Med Princ Pract 24(2): 165-170.
- Dahdouh S, Andescavage N, Yewale S, Yarish A, Lanham D, et al. (2018) In vivo placental MRI shape and textural features predict fetal growth restriction and postnatal outcome. J Magn Reson Imaging 47(2): 449-458.
- Sakov KM, Emerson JW, Campbell KH, Galerneau F, Anders AM, et al. (2018) Estimated Placental Volume and Gestational Age. Am J Perinatol 35(8): 748-757.
- Quant HS, Sammel MD, Parry S, Schwartz N (2016) Second-trimester 3-dimensional placental sonography as a predictor of small-for-gestational-age birth weight. J Ultrasound Med 35(8): 1693-1702.
- Roth CJ, Haeussner E, Ruebelmann T, Koch FV, Schmitz C, et al. (2017) Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space - a pilot study. Sci Rep 7(1): 40771.
- Herzog EM, Eggink AJ, Reijnierse A, Kerkhof MA, de Krijger RR, et al. (2017) Impact of early- and late-onset preeclampsia on features of placental and newborn vascular health. Placenta 49: 72-79.
- Sharony R, Eran K, Biron Shental T, Kidron D (2016) Morphometric characteristics of the umbilical cord and vessels in fetal growth restriction and pre-eclampsia. Early Hum Dev 92: 57-62.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...