
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Research Article(ISSN: 2637-4544) 
Immune Biomarkers for Predictability of Preeclampsia Clinical Scenarios Volume 4 - Issue 1
Ahmed Hagras1* and Maha MHagras2
- 1Obstetrics and Gynecology Department, Faculty of Medicine, Tanta University, Tanta, Egypt
- 2Clinical Pathology Department, Faculty of Medicine, Tanta University, Tanta, Egypt/li>
Received:September 03, 2019; Published: September 30, 2019
Corresponding author: Ahmed Hagras, Lecturer of Obstetrics and Gynecology, Obstetrics and Gynecology Department, Faculty of Medicine, Tanta University, Tanta, Egypt
DOI: 10.32474/IGWHC.2019.03.000176
Abstract
Background: Preeclampsia a chief clinical scenario in obstetric medicine, having highly morbid and grave sequalae particularly if rapidly progressive and left unmanaged. The complexity of immune system components involvement is integrated in the preeclampsia pathology as immune based pathology is one of the cornerstone theories for preeclampsia development that have raised the issue that immune dysfunction affection is an issue that could be investigated in recent research efforts to reveal any immunological biomarker that could be implemented as a predictability tool for preeclampsia development and severity.
Aim: To investigate the possible usage of immune biomarkers in prediction of preeclampsia..
Methodology: The current study was conducted at Departments of Obstetrics & Gynecology and Clinical Pathology, Tanta University Hospitals. A prospective clinical research trial that involved cases having an ongoing gestation below 16 gestational weeks of with maternal age range of 18 to 40 years old were recruited in the current research study. During the study period, a total of 200 research study subjects. Peripheral blood was collected between 5th and 16th gestational weeks calculating the gestational age based on the last menstrual period and crown-rump length using a first trimester sonographic scan cases were clinically and investigationally followed till time of delivery and clinical research data was collected and tabulated for analysis the following was conducted on collected blood samples.
Results: The comparative statistical analysis women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines in which there was no statistical significant difference between both research groups as regards EGF, EOTAXING-CSF,GM-CSF ,IFN ALPHA 2,IFN-GAMMA , IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL17-A, IL-1RA, IL-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IP-10, MIP-1 beta, TNF- alpha, TNF-beta, VEGF (p values=0.812, 0.315, 0.712, 0.635, 0.511, 0.865, 0.829, 0.873, 0.603, 0.807, 0.865, 0.512, 0.925, 0.812, 0.512,0.310,0.423 ,0.478, 0.829, 0.903 ,0.865, 0.314, 0.885, 0.489,0.958,0.288 consecutively) however there was highly statistically significantly higher levels of IL-8 among the preeclampsia research group, and statically significantly higher levels of MCP-1 among no preeclampsia research group (p value= 0.012), whereas there was statically significantly higher levels of MIP-1 alpha among preeclampsia research group (p value= 0.032).
Conclusion: The current study findings denote that there is a possible clinical value in using immune biomarkers in preeclampsia screening, however future research efforts are required to verify the current study results in order to elucidate more clearly the most useful immune cell ratios and inflammatory mediators that could be clinically applicable and useful with the highest sensitivity in order to upgrade the management protocols of preeclampsia.
Introduction
Preeclampsia a chief clinical scenario in obstetric medicine, having highly morbid and grave sequalae particularly if rapidly progressive and left unmanaged .Various theories have aroused to explain the pathophysiological origin and development of preeclampsia one of the cornerstone factors that raise the morbidity is endothelial dysfunction that raises the blood pressure levels besides causing multisystem affection causing multiple pathological affection such as HELLP syndrome [1-4].
Placental immunological tolerance to the maternal immunological system is considered one of the research debate and mystery since it is considered the safeguard against fetal immune rejection as an allograft. The complexity of immune system components involvement is integrated in the preeclampsia pathology as immune based pathology is one of the cornerstone theories for preeclampsia development that have raised the issue that immune dysfunction affection is an issue that could be investigated in recent research efforts to reveal any immunological biomarker that could be implemented as a predictability tool for preeclampsia development and severity [5-8]. Furthermore it was observed that the physiological process of placentation is affected by the maternal immune system activity and the cross talk between the maternal immune system and developing fetal antigens that interact in a manner that is not fully understood .even the process of implantation is affected by products and activity on the immune system such as leukemia inhibitory factor that raise the interest to investigate the normal and abnormal immunological adaptation in pregnancy in correlation to arousal of various disease pathology such as preeclampsia and other auto immune disease [10-12]. The cellular level and molecular level complex mechanisms that because poor trophoblastic invasion is an issue that is not fully revealed although it is highly hypothesized to be immune mediated. an ischemic placenta could in addition cause the release of inflammatory mediators that could be the triggering factor for the pathophysiological development of the preeclampsia disease causing changes in the maternal immune system cellular and molecular profile [13,14]. Prior research groups at molecular and cellular levels have hypothesized that immunological aberrations at functional performance activities of the placental microenvironment could be the chief factor affecting the presentation and prognosis of preeclampsia and the gestational age of onset [15,16]. Interestingly it was previously demonstrated that normal gestation have a clear shift toward a Th2 immuno-tolerant fashion within the peripheral circulation and at the fetal-maternal cellular and molecular cross talk furthermore in an interesting manner it was revealed and displayed by researchers that preeclampsia pathological immune behavior is characteristically featured by Th1 predominance with pro-inflammatory state and Th1/Th2 imbalance besides it was shown that the imbalance and disproportion of T helper cells activities and numbers id one of the dominate immune cellular features in preeclampsia furthermore preeclampsia is correlated to is a global pro-inflammatory systemic environment having raised levels of pro-inflammatory cytokines, chemokines and adhesion molecules within the maternal vascular system interacting in manner that results in excessive systemic inflammatory response and endothelial systemic dysfunction [17-19].
Methodology
The current study was conducted at Departments of Obstetrics & Gynecology and Clinical Pathology, Tanta University Hospitals. A prospective clinical research trial that involved cases having an ongoing gestation below 16 gestational weeks of with maternal age range of 18 to 40 years old were recruited in the current research study. During the study period, a total of 200 research study subjects. Peripheral blood was collected between 5th and 16th gestational weeks calculating the gestational age based on the last menstrual period and crown-rump length using a first trimester sonographic scan cases were clinically and investigationally followed till time of delivery and clinical research data was collected and tabulated for analysis the following was conducted on collected blood samples.
Flow Cytometry
To determine lymphocyte subcategories, peripheral blood have been collected in in test tubes having sodium heparin and stained with monoclonal antibodies against cell surface markers. Blood have been incubated using antibody cocktails for 30 min at room temperature and then diluted in wash buffer. After one centrifugation cycle, investigators used the combination of Immuno- Lyse and Fixative (Beckman Coulter) to wash red blood cells and fix white blood cells. Cells were analyzed using a using a flow cytometer with implementing immunophenotyping using monocloncal antibodies.
Intracellular cytokine analysis
After the incubation, the cells were washed and labeled with monoclonal antibodies to detect intracellular cytokines, samples were analyzed using a flow cytometer.
Luminex assay
Peripheral blood has been collected in serum separator tubes and spun within 2 hours for serum isolation, samples were frozen and stored at −80 °C until the analysis. Cytokines and chemokines, e.g. EGF, GCSF, GM-CSF, IFN-α2, IFN-γ, IL-1α, IL-1β have been assayed using a multiplex assay kit.
Research outcome measures
Clinical and laboratory research data have been collected and analyzed relying on the gestational clinical outcome, e.g. preeclampsia, preterm and gestational diabetes.
Statistical Analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. Data were presented as mean and standard deviations for quantitative data with parametric distribution and median with Inter-Quartile Ranges (IQR) with non-parametric distribution and numbers and percentages for qualitative data. The comparison between two groups was done using independent t-test for quantitative data and Chi-square test for qualitative data and Fisher exact test was used instead of chi-square test when the expected count was found less than 5 in any cell. The comparison between two groups regarding quantitative data was done by using Independent t-test and/ or Mann-Whitney test according to distribution of data. Receiver Operating Characteristic Curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and Area Under Curve (AUC). The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
Results
Table 1 reveals and displays the comparative analysis between women with and without pre-eclampsia regarding demographic data, characteristics and risk factors in which there was no statistical significant difference between preeclampsia and no preeclampsia research groups as regards age, gestational age at blood collection, BMI, gravidity, term deliveries, preterm deliveries, abortions, lived births, smoking, auto immune disease, chronic renal disease previous history of preeclampsia, multifetal gestations, pregestational DM, chronic hypertension (p values =0.731, 0.336, 0.279, 0.516, 0.627, 0.711, 0.452, 0.350, 0.568, 0.480, 0.732, 0.170, 0.337, 0.210, 0.369 consecutively).
Table 1: Comparison between women with and without pre-eclampsia regarding demographic data, characteristics and risk factors.
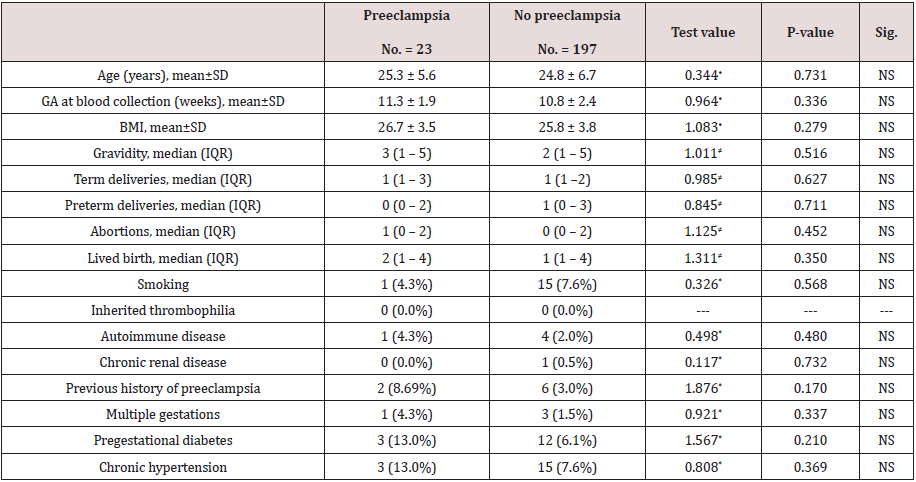
•: Independent t-test; ≠: Mann-Whitney test; *: Chi-square test
Table 2 reveals and displays the comparative statistical analysis between cases with and without pre-eclampsia regarding pregnancy outcomes in which preeclampsia research group had statistically significantly lower gestational age at delivery in comparison to no preeclampsia research group (p values <0.001 ) ,besides there was statically significantly higher systolic and diastolic blood pressure readings among the preeclampsia research group (p values<0.001) preterm labor was statically significantly higher among the no preeclampsia research group (p value<0.001).furthermore there was no statically significant difference as regards APGAR scoring at 1,5min,cesarean section delivery rates small for gestational age, fetal demise, gestational DM (p values= 0.314,0.521,0.061, 0.072,0.063, 0.568 consecutively).
Table 2: Comparison between cases with and without pre-eclampsia regarding pregnancy outcomes.
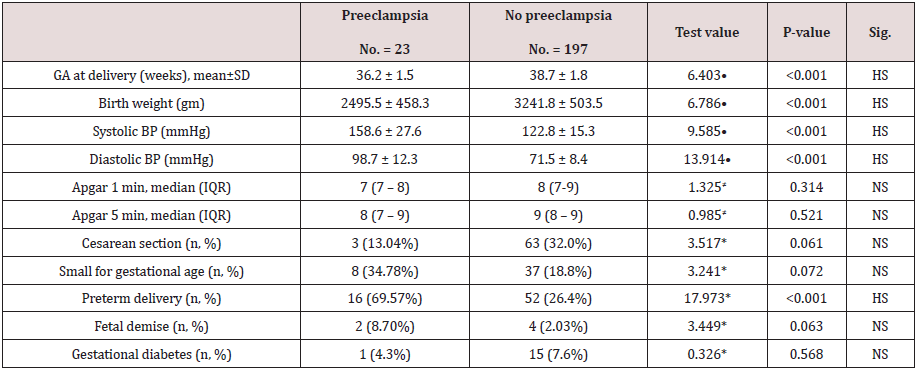
•: Independent t-test; ≠: Mann-Whitney test; *: Chi-square test
Table 3 reveals and displays the comparative statistical analysis between women with and without pre-eclampsia regarding immune of peripheral blood in which there was no statically significant difference as regards %CD3+ (T cells), %CD3+ CD4 (T helper cells), % CD3+CD8+ (T cytotoxic cells), % CD3+CD56+ (NKT cells), % CD3−CD56+ (NK cells), % CD3−CD56+CD16+ (Cytotoxic NK cells), %CD19+ (B cells), %CD19+CD5+ (B cells) (p values =0.234, 0.786, 0.467, 0.737, 0.308, 0.691, 0.208, 0.375 consecutively), on the other hand there was statically significantly lower %CD4+ CD25bright CD127dim/- (Treg cells) among the no preeclampsia research group in comparison to preeclampsia research group (p value<0.001).
Table 3: Comparison between women with and without pre-eclampsia regarding immune of peripheral blood.
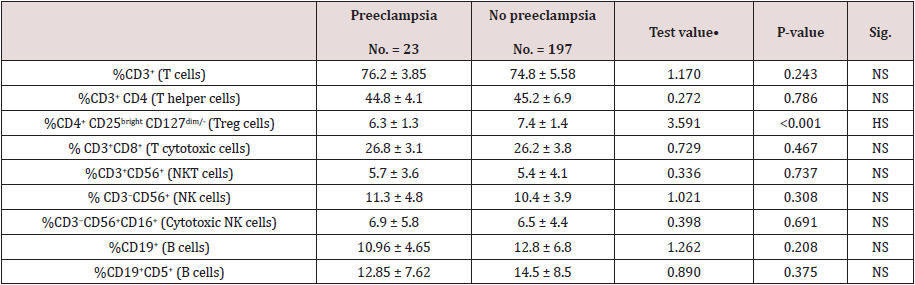
•: Independent t-test
Table 4 reveals and displays the comparative statistical analysis women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines in which there was no statistical significant difference between both research groups as regards EGF, EOTAXING-CSF,GM-CSF, IFN ALPHA 2, IFN-GAMMA, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL17-A, IL-1RA, IL-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IP-10, MIP-1 beta, TNF- alpha, TNF-beta, VEGF (p values=0.812, 0.315, 0.712, 0.635, 0.511, 0.865, 0.829, 0.873, 0.603, 0.807, 0.865, 0.512, 0.925, 0.812, 0.512, 0.310, 0.423, 0.478, 0.829, 0.903, 0.865, 0.314, 0.885, 0.489, 0.958, 0.288 consecutively) however there was highly statistically significantly higher levels of IL-8 among the preeclampsia research group, and statically significantly higher levels of MCP-1 among no preeclampsia research group (p value= 0.012), whereas there was statically significantly higher levels of MIP-1 alpha among preeclampsia research group (p value= 0.032).
Table 4: Comparison between women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines.
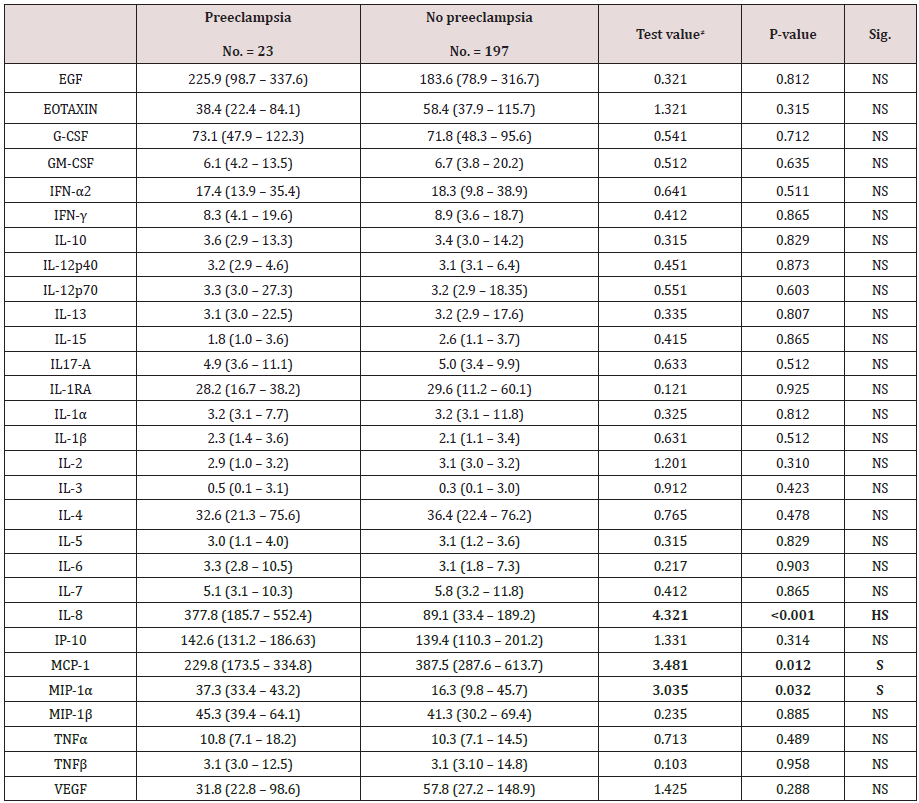
≠: Mann-Whitney test
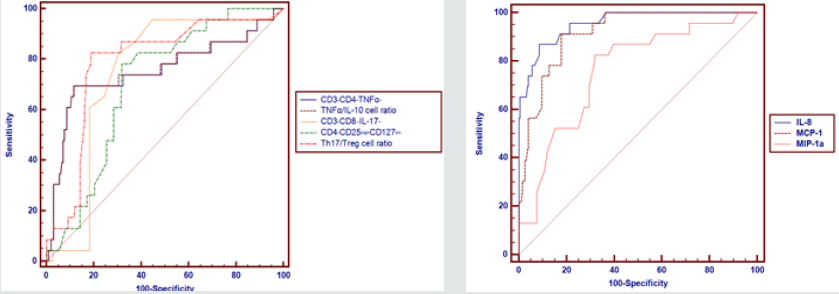
Table 5 and Figures 1 & 2 reveal and display the Receiver operating characteristic curve (ROC) for the independent predictors of preeclampsia among the research study subjects in which CD3+CD4+TNFα+, TNFα/IL-10 cell ratio,CD3+CD8−IL- 17+,CD4+CD25brightCD127dim,Th17/Treg cell ratio,IL-8,MCP- 1,MCP-1a had cut off points >50.75, >49.83,>1.18,≤ 6.5, >0.287, >224, ≤260, >30 consecutively that had AUC =0.753, 0.755, 0.746, 0.703, 0.786, 0.955, 0.916, 0.76 consecutively, and statistical sensitivity= 69.57, 69.57, 82.61, 78.26, 82.61, 86.96, 91.3, 82.61 consecutively, overall indices have shown that IL-8 at cutoff value >224, AUC =0.955, SENSITIVITY = 86.96, specificity = 91.37. Positive predictive value= 54.1 Negative predictive value = 98.4 is the most effective immune biomarker for preeclampsia prediction.
Table 5: Preeclampsia among the research study subjects.
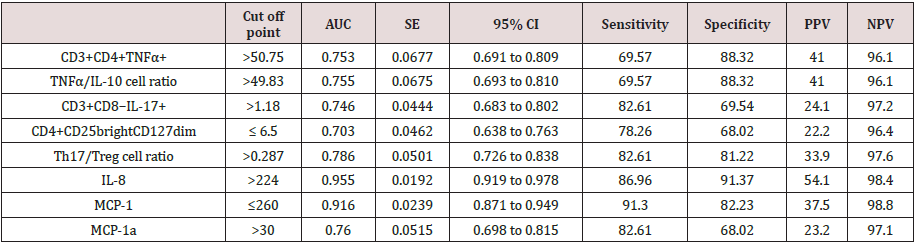
AUC: Area under curve; SE: Standard error; 95% CI: 95% Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Figure 1 & 2: Receiver Operating Characteristic Curve (ROC) for the independent predictors of preeclampsia among the research study subjects.
Discussion
Prior research studies and efforts have observed that there is a possible linkage between immunological system cells and agents secreted and the trigger of preeclampsia pathophysiological course of development .preeclampsia as a disease of theories have raised the research interest and debate among various investigators in which they have tried to observe the pathophysiological origin and causative issues at molecular and cellular levels to observe the best immune predictability biomarker ,since early disease prediction and management would markedly improve the clinical outcomes of the disease [1,3,9,13].
A prior research study like the current 5 study in approach and methodology have revealed a displayed among its research study findings that there is a statistically significant correlations and linkage between peripheral blood immune effectors, serum levels of cytokines and chemokines in early pregnancy and preeclampsia development making the usage of the immune biomarkers a useful predictability [2,7,15].
Another research team of investigators have observed that a predominant Th1 immunological system profile is correlated to a pro-inflammatory immunological response, endothelial dysfunction and poor placentation all relevant findings in preeclampsia clinical scenarios (besides an elevated percentage of Th1 cells and Th1/Th2cell ratios in peripheral circulation was observed in preeclampsia hematological pathological changes in comparison to normal gestations interestingly in harmony and great similarity to the current study findings it was previously demonstrated that the proportion of T helper cell expression is statically significantly higher in cases having preeclampsia in comparison to normal pregnancies [4,7,18].
Raised percentages of Th17 and reduced percentages of Treg cell categories have been observed by prior investigators in peripheral circulation of preeclamptic cases. Furthermore, prior research efforts investigating the cytokine and chemokine activities have observed that raised serum levels of IL-8 and MIP- 1α and the reduced level of MCP-1 within early gestation is correlated and linked to pathological course of preeclampsia development [8,17].
Conclusions and Recommendations
The current study findings denote that there is a possible clinical value in using immune biomarkers in preeclampsia screening, however future research efforts are required to verify the current study results in order to elucidate more clearly the most useful immune cell ratios and inflammatory mediators that could be clinically applicable and useful with the highest sensitivity in order to upgrade the management protocols of preeclampsia. Future research studies should be implemented in multicentric fashion taking in consideration the possible coexisting medical diseases with preeclampsia such as gestational DM and autoimmune diseases that could have an influence on the course of the disease and clinical expressive pattern.
References
- Ananth CV, Keyes KM, Wapner RJ (2013) Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 347: f6564.
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, et al. (2013) Born Too Soon: the global epidemiology of 15 million preterm births. Reprod Health 10 (Suppl 1): S2.
- Toldi G, Saito S, Shima T, Halmos A, Veresh Z, et al. (2012) The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol 68(2): 175-180.
- Toldi G, Vasarhelyi ZE, Rigo J, Orban C, Tamassy Z, et al. (2015) Prevalence of regulatory T-cell subtypes in preeclampsia. Am J Reprod Immunol 74(2): 110-115.
- Sahin S, Ozakpinar OB, Eroglu M, Tulunay A, Ciraci E, et al. (2015) The impact of platelet functions and inflammatory status on the severity of preeclampsia. J Matern Fetal Neonatal Med 28(6): 643-648.
- Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM (2013) The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertension 3(1): 44-47.
- Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, et al. (2014) The classification: diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 4(2): 97-104.
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R (2014) Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10(8): 466-480.
- Poon LC, Nicolaides KH (2014) Early prediction of preeclampsia. Obstet Gynecol Int 2014: 297397.
- Rahimzadeh M, Norouzian M, Arabpour F, Naderi N (2016) Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol 12(2): 209-227.
- Cui S, Gao Y, Zhang L, Wang Y, Zhang L, et al. (2016) Combined use of serum MCP-1/IL-10 ratio and uterine artery Doppler index significantly improves the prediction of preeclampsia. Clin Chim Acta 473: 228-236
- Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, et al. (2012) The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol 93(2): 75-81
- Forest JC, Charland M, Masse J, Bujold E, Rousseau F, et al. (2012) Candidate biochemical markers for screening of pre-eclampsia in early pregnancy. Clin Chem Lab Med 50(6): 973-984.
- Lau SY, Guild SJ, Barrett CJ, Chen Q, Mccowan L, et al. (2013) Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-Analysis. Am J Reprod Immunol 70(5): 412-427.
- Mol BW, Roberts CT, Thangaratinam S, Magee LA, DE Groot, CJ Hofmeyr, et al. (2016) Pre-eclampsia. Lancet 387(10022): 999-1011.
- Mosimann B, Wagner M, Poon LCY, Bansal AS, Nicolaides KH, et al. (2013) Maternal serum cytokines at 30-33 weeks in the prediction of preeclampsia. Prenat Diagn 33(9): 823-830.
- Park HJ, Shim SS, Cha DH (2015) Combined screening for early detection of preeclampsia. Int J Mol Sci 16(8): 17952-17974
- Ribeiro VR, Romao-Veiga M, Romagnoli GG, Matias ML, Nunes PR, et al. (2017) Association between cytokine profile and transcription factors produced by T cells subsets in early and late-onset preeclampsia. Immunology 152(1): 163-173.
- Roberts JM, Bell MJ (2013) If we know so much about preeclampsia, why haven't we cured the disease? J Reprod Immunol 99(1-2): 1-9.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





