Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
Validity of Serum Markers for Fibrosis Staging in Chronic Hepatitis B and C Patients Volume 3 - Issue 1
Irfan Ahmad*
- Government Medical College, Srinagar, India
Received: November 11, 2020; Published: December 11, 2020
*Corresponding author: Irfan Ahmad, Government Medical College, Srinagar, India
DOI: 10.32474/CTGH.2020.03.000154
Introduction
According to latest report globally about 350 million people are affected by Hepatitis B virus and about 686,000 die every year from Hepatitis B related diseases[1,2]. Similarly, more than 185 million people around the world are infected with Hepatitis C virus, of whom 350,000 die each year[3,4]. Hepatic fibrosis, regardless of the underlying a etiology, is a consequence of accumulation of extracellular matrix components in the liver. This process is caused by persistent liver damage and consequent wound healing reaction, leading to cirrhosis, portal hypertension, and hepatocellular carcinoma (HCC), all these cumulatively leading to increased morbidity and mortality [5,6]. Thus accurate assessment of liver fibrosis is essential for successful individualized disease management for people with chronic hepatitis B and C7. Although liver biopsy, till date, remains the gold standard for diagnosis of liver fibrosis but it is far from optimal because of many associated complications [8,9,10]. To overcome these limitations multiple noninvasive modalities have been introduced. Various non-invasive parameters which could replace the biopsy of the infected liver include: - FIB-4[11], APRI[12], AST/ALT ratio[13], Kings Score[14], Frons index[15], Elastography[16] and Fibro scan[17]. Our study included the following three parameters as alternative to liver biopsy: FIB-4, APRI (Aspartate Aminotransferase to Platelet Ratio Index) and AST/ALT Ratio (Aspartate transaminase)/ (Alanine transaminase).
Materials and Methods
The current study was a hospital based prospective study which was conducted in the Department of Internal Medicine and Department of Gastroenterology, Government Medical College, Srinagar, J&K (INDIA). The study was approved by the respective ethical committees of the college. The study was conducted over a period of 36 months starting from August 2014 to July 2017. The study was approved by the respective ethical committees of the college. A total of 262 patients were included and out of them 172 were infected with hepatitis B and 90 patients were infected with hepatitis C. All Chronic Hepatitis B (CHB) and Chronic Hepatitis C (CHC) patients seen in the OPD or admitted in IPD were enrolled and investigated as per the study design. Patients were explained about the liver biopsy procedure, its advantages and possible adverse effects. Patient’s history was taken, and physical examination was carried out. Written informed consent was obtained from each participant. Patients were ≥ 18 years of age of either sex. All patients’ laboratory data (alanine aminotransferase [ALT], aspartate aminotransferase [AST], platelet count) were collected. FIB-4[12], APRI[13]were calculated by sterlings[11]and wai’s[12] formulas respectively:
(* where ULN = upper limit of normal for that laboratory)
Fibrosis stage was calculated by abstraction from liver biopsy reports. Fibrosis scores from different scoring systems (IASL[18], Metavir[19], Ishak[20], Knodell[21]) were mapped to a F0–F4 equivalency scale: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis (Table 1). If the patient had more than one biopsy, the earliest biopsy with the highest fibrosis stage and available laboratory results was used for this analysis[18].
Table 1. Mapping of fibrosis stages from histological classification systems to a common F0–F4 scale. IASL: International Association for the study of liver.
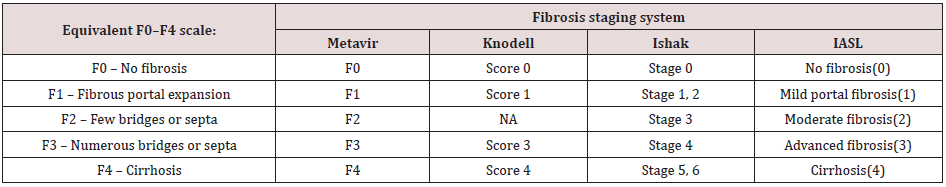
Mapping of fibrosis stages from histological classification systems to a common F0–F4 scale. IASL: International Association for the study of liver.
Statistical Analysis
We first validated predefined serum markers then developed and validated cut-offs of the serum markers for classification of cirrhosis (F4) or advanced fibrosis (F3–F4). The serum markers of interest were as follows: APRI, FIB-4 and ALT/AST ratio, as defined in the previous section. The endpoints of interest were the presence of advanced fibrosis (F3–4 vs F0–2) and the presence of cirrhosis (F4 vs F0–3) in case of Chronic Hepatitis C. However, in our study, in patients with chronic hepatitis B infection who underwent liver biopsy, no patient had a histological grade of F3 or F4. In such scenario, comparison was made between those with few bridges or septa (F2) and no fibrosis (F0). The data was entered in Microsoft Excel Spreadsheet. Continuous variables were summarized as mean and standard deviation (SD). Categorical variables were summarized as percentages. Radius of Curvature (ROC) curves were constructed for APRI and FIB-4 scores and AST/ ALT ratio. Liver biopsy results was taken as standard. Area under ROC curve was reported along with its 95% confidence interval for APRI, FIB-4 and AST/ALT ratio. A p-value of <0.05 was considered as statistically significant.
Results
Out of 90 CHC patients, 18 belonged to 18-30-year age group, 36 patients belonged to 31-45 age group. Twenty-three patients were between 46-60 years of age whereas thirteen patients belonged to 61-75-year age group. Similarly, out of 172 CHB, 34 belonged to 18-30-year age group, 68 patients belonged to 31-45 age group. Fifty-five patients were between 46-60 years of age whereas fifteen patients belonged to 61-75-year age group. In our study out of 172 patients of CHB, 107 were male and 65 were females with male to female ratio of 1.6:1. Similarly out of 90 patients of hepatitis CHC, 56 were males and 34 were females and the male to female ratio was 1.6:1. On combining the two groups, out of 262 patients there was slight male preponderance with total male to female ratio of 1.6:1.
A. Chronic Hepatitis C
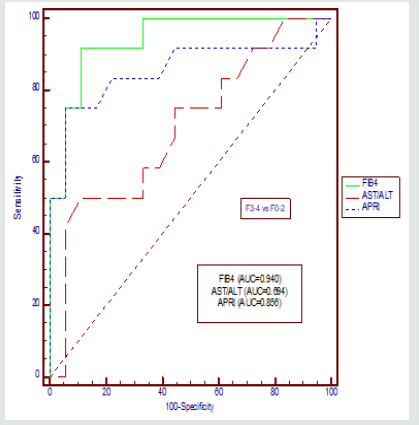
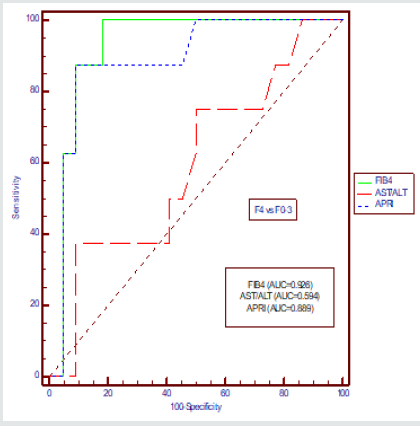
We compared AUROCs of the FIB-4 index with those of the other indices for the classification of advanced fibrosis and cirrhosis, respectively (Figure-1&2, Table 2&3).
Table 2. Comparison of ROC curves [F3-4 vs F0-2 among CHC study participants]. *Statistically Significant Difference (P-value<0.05).

Table 3. Comparison of ROC curves [F4 vs F0-3 among CHC study participants]. *Statistically Significant Difference (P-value<0.05).

The AUROC for FIB-4 in differentiating F3–F4 from F0–F2 was 0.940 (95% CI: 0.788–0.994) when compared with AST/ALT Ratio with p value of 0.019 and AUROC for APRI was 0.856(95% CI: 0.680–0.957) when compared with FIB-4 with p value of 0.321 for CHC. The AUROC for FIB-4 in differentiating F4 from F0–F3 was 0.926 (95% CI: 0.769–0.989) when compared with AST/ALT Ratio with p value of 0.007 and AUROC for APRI was 0.070(95% CI: 0.721–0.974)) when compared with FIB-4 with p value of 0.374 for CHC.
B. Chronic Hepatitis B
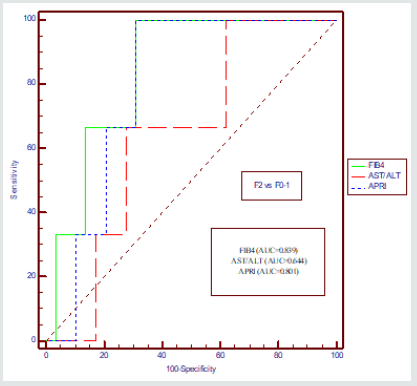
In case of CHB, comparison was done between AUROCs of the FIB-4 index with those of the other indices for the classification of those with few bridges or septa (F2) and no fibrosis (F0); and fibrous portal expansion (F2 vs F0-F1)(Figure-3,Table 4). The AUROC for FIB-4 in differentiating F2 from F0–F1 was 0.839 (95% CI: 0.667–0.944) when compared with AST/ALT Ratio with p value of 0.038 and AUROC for APRI was 0.801(95% CI: 0.614– 0.915) when compared with FIB-4 with p value of 0.732 for CHB.
Table 4. Comparison of ROC curves [F2 vs F0-1 among CHB study participants]. *Statistically Significant Difference (P-value<0.05).

Cut-off Values for Predicting Fibrosis and Cirrhosis Using FIB-4 in CHC
Based on the AUROC analysis in the previous section, FIB-
4 had the best overall utility for prediction of advanced fibrosis
and cirrhosis in a chronic hepatitis C population compared with
the other two markers, as FIB-4 score outperformed the other
serum markers and was superior to AST/ALT ratio and almost
similar to APRI. We next derived optimal cut-off values of FIB-4
for distinguishing the lower end of liver stage (F0–F2) and upper
end of liver stage (F3, cirrhosis) for CHC. The optimal cut-off of
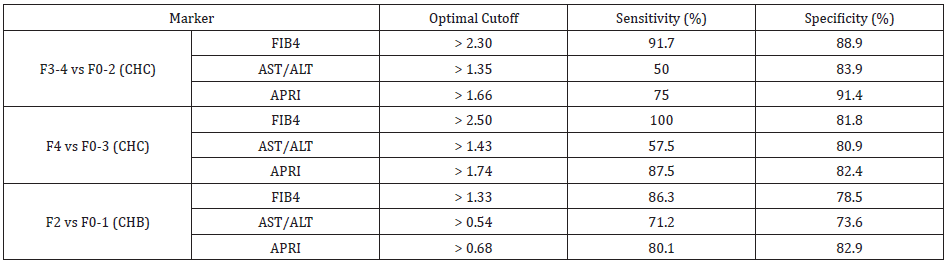
FIB-4 in distinguishing F3,F4 vs F0-F2 was >2.30 with sensitivity
and specificity of 91.7% and 88.9% respectively. For APRI, optimal
cut-off was >1.66 with sensitivity and specificity of 75% and 91.4%
respectively and for AST/ALT ratio optimal cut-off was >1.35 with
sensitivity and specificity of 50% and 83.9% respectively.
Similarly, the optimal cut-off of FIB-4 in distinguishing F4 vs F0-
F3 was >2.50 with sensitivity and specificity of 100% and 81.8%
respectively. For APRI, optimal cut-off was >1.74 with sensitivity
and specificity of 87.5% and 82.4% respectively and for AST/ALT
ratio optimal cut-off was >1.43 with sensitivity and specificity of
57.5% and 80.9% respectively.
Cut-off Values for Predicting few bridges or septa (F2) and no fibrosis (F0); and fibrous portal expansion (F2 vs F0-F1) in CHB
Similarly, the optimal cut-off of FIB-4 in distinguishing few bridges or septa (F2) and no fibrosis (F0); and fibrous portal expansion (F2 vs F0-F1) was >1.33 with sensitivity and specificity of 86.3% and 78.5% respectively. For APRI, optimal cut-off was >0.68 with sensitivity and specificity of 80.1% and 82.9% respectively and for AST/ALT ratio optimal cut-off was >0.54 with sensitivity and specificity of 71.2% and 73.6% respectively[Table 5].
Discussion
While analysing our results for these markers, FIB-4 was
found out to be a better marker for detecting the degree of fibrosis
in CHC. In early stages of fibrosis (F0-F2), keeping a cut-off value
>2.3, the sensitivity and specificity of FIB-4 was 91.7% and 88.9%
respectively. At further advanced degree of fibrosis i.e., cirrhosis,
the sensitivity of cut-off value of >2.5 for FIB-4 reaches 100%.
The cut-off valve obtained of APRI score 1.66 was similar to
study conducted by [22]where the cut-off valve of APRI has been
1.5, the sensitivity and specificity of APRI has been more than
75% and 91.4% for F0-F2 and F3, F4 (significant fibrosis) score.
The APRI score in similarity with FIB-4 score is quite valuable in
advanced stage of fibrosis (cirrhosis), but in early stages of fibrosis
the APRI score has not been of much significance, though the effect
can be attributed to very less number of patients in this group. The
higher sensitivity and specificity pattern obtained from FIB-4 and
APRI score was not reflected by AST/ALT ratio in our study, with the
sensitivity and specificity falling to 50% and 37% to stages F3 and
F4 respectively. The superiority of FIB-4 and APRI with AST/ALT
ratio in predicting the fibrosis is validated by the study conducted
by [23]. However in case of CHB,FIB-4 has definitely being shown
to be almost close to liver biopsy in predicting the histopathology
of liver. The other two scoring systems like APRI and AST/ALT ratio
have not proved to be better than FIB-4. The results of the study
conducted by Yilmaz et al. [24] reported that APRI had acceptable
accuracy for assessment of fibrosis with CHC but same was not
applicable for CHB, though we had no patients of advanced fibrosis,
but even on comparing the patients of mild with moderate fibrosis,
APRI was not as significant marker with AUC of 0.644 and 95%
CI of 0.455 to 0.804. FIB-4 again served as a significant marker to
distinguish patients of mild fibrosis (F0, F1) from patients with
moderate fibrosis with AUC of 0.839 and 95% CI (0.667 to 0.944)
and p-valve of 0.038. The studies conducted by Zhang et al. [25]
in the past revealed FIB-4 as a diagnostic marker to discriminate
between patients of early fibrosis and advanced fibrosis.
The advantage of our study was its prospective design and
adoption of strict inclusion and exclusion criteria. The disadvantage
of our study was the small sample size.
Conclusion
Thus, our study suggests that FIB-4 and APRI are excellent surrogate markers for liver fibrosis while ASL/ALT is not a very sensitive marker. Among all these scores FIB-4 is the best. On the basis of our results, we recommend use of FIB-4 as a surrogate marker for liver fibrosis across all age groups. We further recommend that larger number of patients to be undertaken in future studies.
References
- Vasilios Papastergiou Rosa, Lombardi Douglas, MacDonald Emmanuel, A Tsochatzis (2015) Global Epidemiology of Hepatitis B Virus (HBV) Infection. Current Hepatology Reports 14(3):171-178.
- Lancet (2015) Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013 Mortality and Causes of Death Collaborators. 385(9963):117-171.
- Mohd Hanafah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: New estimates of age specificantibody to HCV seroprevalence. Hepatology 57(4):1333-1342.
- Lavanchy D (2009) The global burden of hepatitis C. Liver Int 29(Suppl 1): 74-81.
- Friedman SL (1998) in Diseases of the Liver, eds Schiff E, Sorrell M, Maddrey W (Lippincott-Raven, Philadelphia) 8th Ed. pp 371-386.
- Ikeda K, Kawada N, Wang YQ, Kadoya H, Nakatani K, etal. (1998) Expression of Cellular Prion Protein in ActivatedHepatic Stellate Cells. Am J Pathol 153(6):1695-1700.
- Baranova A, Lal P, Birerdinc A, Younossi ZM (2011) Non-invasive markers for hepatic fibrosis. BMC Gastroenterol 11.
- Seeff LB1, Everson GT, Morgan TR, Curto TM, Lee WM, etal. (2010) Complication rate of percutaneous liver biopsie among persons with advanced chronic liver disease in the HALT-C trial.Clin Gastroenterolo Hospital8(10): 877-883.
- Regev A, Berho M, Jeffers LJ Milikowski C, Molina EG, etal. (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol97(10):2614-2618.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy. Hepatology 49(3):1017-1044.
- Smith JO, Sterling LK (2009) Systematic review: Non-invasive methods of fibrosis analysis in chronic hepatitis C.Aliment Pharmacol Ther 30(6): 557-576.
- Chun-Tao Wai, Joel K Greenson, Robert J Fontana, John D Kalbsfleisch, Jorge A Marrero, etal. (2003) A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38(2): 518-526.
- Williams AL, Hoofnagle JH (1988) Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterolog 95(3): 734-739.
- Cross TJ1, Rizzi P, Berry PA, Bruce M, Portmann B, etal. (2009) King's Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 21(7): 730-738.
- Forns X, Ampurdanes S, Llovet JM, Aponte J, Martinez-Bauer E, etal. (2002) Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model.Hepatology36: 986-992.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy.Hepatology 49: 1017-1044.
- Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, etal. (2005) Prospective comparison of transient Elastography, Fibrotest, APRI and liver biopsy for the assessment of fibrosis in Chronic hepatitis C.Gastroenterology128(2): 343-350.
- J Li SC Gordon, LB Rupp, T Zhang, JA Boscarino, V Vijayadeva, etal. (2014) The validity of serum markers for fibrosis staging in chronic hepatitis B and C.J Viral Hepat 21(2): 930-937.
- Hepatology (1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. 20(1): 15-20.
- Kamal Ishak, Amelia Baptista, Leonardo Bianchi, Francesco Callea, Jan De Groote, etal. (1995) Histological grading and staging of chronic hepatitis.J Hepatol22(6): 696-699.
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, etal. (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1(5): 431-435.
- H Shehab, I Elattar, T Elbaz, M Mohey, G Esmat (2014) CUFA algorithm: assessment of liver fibrosis using routine laboratory data. Journal of ViralHepatitis 21(12): 956-996.
- Holmberg SD, Lu Mei, Rupp LB, Lamerato LE, Moorman AC, etal. (2013) Non-invasive serum fibrosis Markers forscreening and staging chronic hepatitis C virus patients in a large US cohort.Clinical Infect Dis 57(2): 240-246.
- Yusuf Yilmaz, Oya Yonal, Ramazan Kurt, Muharrem Bayrak, Bilge Aktas, etal. (2011) Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease. Hepat Mon 11(2): 103-106.
- Zhang YF, Shi H, Chen LB, Xu QH (2010) Value of FIB-4 for the diagnosis of liver fibrosis in chronic hepatitis B. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 24(3): 215-217.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...