Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
The Risk of Hospitalization due to COVID-19 in Patients with Inflammatory Bowel Disease Volume 3 - Issue 4
Merit Kase1, Clas-Göran AF Björkesten1, Veli-Jukka Anttila2, Jonna Jalanka3, Juuso Arkkila4, Perttu Arkkila1 and Pauliina Molander1*
- 1Abdominal Center, Gastroenterology, Helsinki University Hospital and University of Helsinki, Finland
- 2Department of Infectious Diseases, Helsinki University Hospital and University of Helsinki, Finland
- 3Immunobiology Research Program, Faculty of Medicine, University of Helsinki, Finland
- 4Institute of Dentistry, University of Turku, Finland
Received:March 01, 2022 Published: March 04, 2022
*Corresponding author: Pauliina Molander, Abdominal Center, Gastroenterology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
DOI: 10.32474/CTGH.2022.03.000166
Abstract
Objectives: The novel coronavirus SARS-CoV2 became a worldwide pandemic in 2020. It is known that patients with inflammatory bowel disease (IBD) are at an increased risk of infection, particularly when on immunosuppressive therapy. The outcomes of COVID-19 in IBD patients remain somewhat unclear.
Methods: This Finnish retrospective observational cohort study enrolled 74 patients with an established IBD diagnosis and a confirmed COVID-19 infection. Patient data (age, sex, body mass index, IBD type, biochemical and clinical activity, comorbidities [Charlson comorbidity index [CCI]) and symptoms of COVID-19 were compared with hospitalization due to the COVID-19 infection.
Results: We found that older age (p < 0.01) and comorbidities (CCI score higher than one [p < 0.01]) were associated with hospitalization due to COVID-19 infection. In contrast, none of the studied pharmacological treatments for IBD, IBD type or disease activity were associated with a higher risk of hospitalization.
Conclusion: Our study shows that comorbidities and older age are associated with hospitalization due to COVID-19. On the other hand, different pharmacological treatments for IBD were not linked to a higher risk of hospitalization.
Keywords:Inflammatory bowel diseases; Crohn’s disease; Ulcerative Colitis; COVID-19; Immunosuppressive Treatment
Introduction
The coronavirus pandemic is a worldwide health crisis brought on by severe acute respiratory syndrome coronavirus 2 (SARS CoV 2), which causes a COVID-19 (coronavirus disease 2019) infection [1]. The disease has continued to spread globally and was classified as a pandemic on 11 March 2020, by the World Health Organization [2]. Clinical symptoms in COVID-19 vary between patients, but most individuals have a mild form of the disease with no or flu-like symptoms, including a dry cough, fever, runny nose and fatigue. Additional symptoms may comprise shivering, throat pain, anosmia, headache, joint pain, nausea and diarrhoea [3,4]. In more severe forms of the disease, marked inflammation and progressive pneumonia occur, leading to difficulties in breathing. A COVID-19 infection has often proved to be more severe in patients over 60 years of age. Furthermore, most patients with COVID-19 requiring hospitalization or intensive care unit (ICU) admission have been shown to have at least one comorbidity, such as chronic lung or heart disease, diabetes or conditions that affect their immune system [5]. In addition, smokers have been suggested to develop more severe symptoms of COVID-19 and are more likely to be admitted to intensive care, to need mechanical ventilation or to die than to non-smokers [6].
The treatment of inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), frequently includes immunosuppressant medications [7-10]. The immunomodulators commonly used in IBD are corticosteroids, thiopurines, methotrexate, calcineurin inhibitors, anti-tumour necrosis factor agents or other biologicals. Their modes of action differ from each other, but they all compromise, to some extent, the patient’s immune response [11]. This may increase the patient’s risk of viral and bacterial infections and adverse outcomes of COVID-19 [12,13]. However, published data on possible associations of immunosuppressive therapy with severe COVID-19 remain inconsistent. Data extracted from the international registry Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD; 1,439 cases, 112 with severe COVID-19) suggest an increased risk with thiopurines either combined with biologicals or as a monotherapy [14], whereas data from the French national health system (268,185 IBD patients, 600 hospitalizations) indicate no such association [15]. So far, there is no clear evidence for an increased risk of more severe outcomes in patients with IBD in the context of COVID-19. This study aimed to describe how COVID-19 presents and evolves in patients with IBD and to identify potential risk factors that may predict the severity and outcomes of a COVID-19 infection in IBD patients.
Methods
This was a retrospective, observational cohort study. All eligible patients were adults (18 years and older) with an established diagnosis of CD or UC and a confirmed diagnosis of COVID-19, which was defined as the PCR-confirmed presence of the SARS-CoV-2 genome in a nasopharyngeal swab. The Hospital District of Helsinki and Uusimaa is the largest hospital district in Finland, covering a population of more than 1.7 million. The IBD registry is an integrated platform of the hospital patient data system and comprises 5,194 secondary or tertiary care patients with an IBD diagnosis, treated mostly with immunosuppressants and biologicals. We identified IBD patients with a COVID-19 diagnosis by performing a search combining the hospital district’s COVID-19 registry and the IBD registry. The more detailed patient and disease data were collected retrospectively from the patient electronic charts in April 2021. For all eligible patients, we collected the following data: age, sex, ethnicity, pregnancy, body mass index, IBD type, IBD duration, surgical IBD treatment, pharmacological IBD treatment, other comorbidities (expressed with the Charlson Comorbidity Index [CCI] [16] signs and symptoms of COVID-19 (fever, cough, dyspnoea, dysosmia/dysgeusia, pharyngitis, diarrhoea, arthralgia-myalgia/ asthenia, rhinitis, dysphonia, headache, abdominal pain, nausea/ vomiting, thrombosis), antibiotic and anticoagulant therapies for COVID-19, COVID-19 outcomes (hospitalization on a regular ward and in an ICU as well as death), and smoking status.
Data on faecal calprotectin (FC) as a surrogate marker of inflammation were recorded 0–6 months before the COVID-19 infection, during the COVID-19 infection and after the COVID-19 infection. Values considered to be normal for FC were < 200 μg/g [17,18]. The last registration on clinical activity of IBD was assessed based on patient charts. Clinical disease activity was determined according to the presence or absence of symptoms due to IBD (number of bowel movements, presence or absence of abdominal pain and presence of blood on defecation). The Charlson Comorbidity Index (CCI) is a validated and easily applicable method of estimating the disease severity and the risk of death from a comorbid disease. It has also been shown that a higher mean CCI score is significantly associated with mortality and disease severity in COVID-19 patients [19].
Statistical analysis
Statistical analysis was performed using the R software environment (version R-3.6.2). Differences in the hospitalization status and the studied variables were tested for significance using logistic regression, where the age and BMI of the patients served as confounding factors. Statistical significance was set at p < 0.05. The values are presented as numeric with percentage or as mean with SD.
Ethical considerations
Permission to conduct the study was received from the institutional review board of Helsinki University Hospital. As this was a retrospective, non-interventional patient records review study, no ethics committee approval was required.
Results
Study population
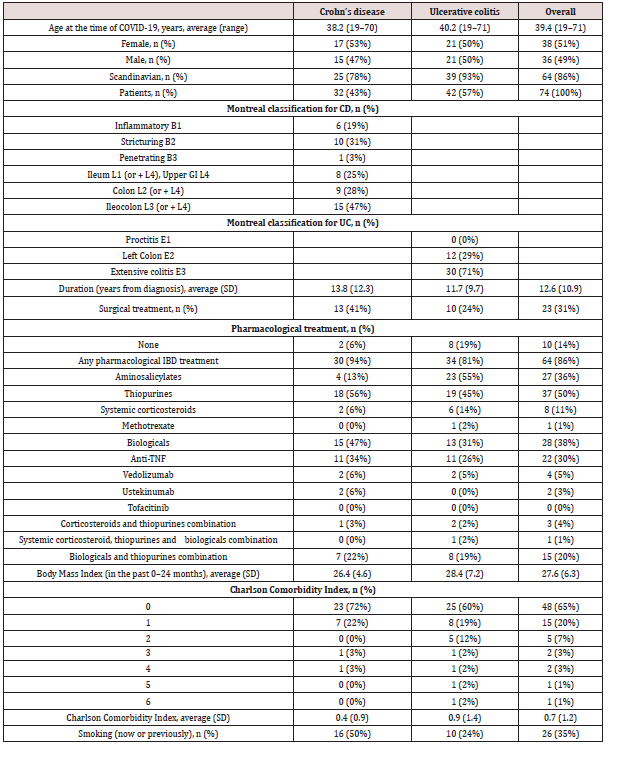
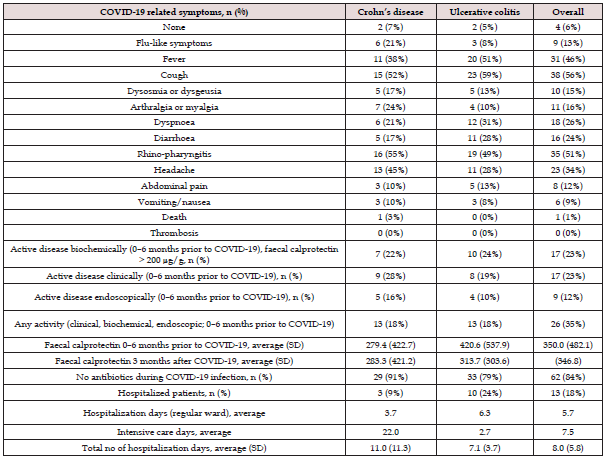
Between 29 January 2020 and 15 April 2021, 74 patients with IBD (CD n = 32 [43%], UC n = 42 [57%]) had been diagnosed with a COVID-19 infection. The patients’ baseline characteristics are shown in (Table 1). Within the six months prior to the COVID-19 infection, 18% (n = 13) of the CD patients and 18% (n = 13) of UC patients had an active IBD. Based on the available data, 22% (n = 7) of the CD and 24% (n = 10) of the UC patients had a biochemically active disease, whereas 28% (n = 9) of the CD and 19% (n = 8) of the UC patients had a clinically active disease. Six percent (n = 2) of the CD patients and 19% (n = 8) of the UC patients were not on any pharmacological treatment for IBD at the time of COVID-19 diagnosis. At the time of COVID-19 diagnosis, three patients (4%) were on systemic corticosteroid and thiopurine combination therapy; of these, only one UC patient was hospitalized on a regular ward and did not require ICU admission. We identified only one patient who was on concomitant medication with systemic corticosteroids, thiopurine and biologicals, and this patient was not hospitalized. Seven CD patients (22%) and eight UC patients (19%) were treated with thiopurine and biologicals, of these, one patient with CD and one with UC were hospitalized on a regular ward. Overall, two patients were pregnant, and neither of them was hospitalized. Nearly threequarters (72%, n = 23) of the CD patients and two-thirds (60%, n = 25) of the UC patients had no significant comorbidities (CCI 0). One comorbidity was present in 22% (n = 7) of the CD patients and in 19% (n = 8) of the UC patients. Hence, only 15% of all patients had two or more comorbidities. Seven patients (9%) had asthma, while none had been diagnosed with chronic obstructive pulmonary disease. For 36 patients, an FC value determined 0–6 months prior to the COVID-19 infection was available: the average value for CD patients was 279 μg/g and for UC patients 421 μg/g (FC range in all patients 5–1,600 μg/g, SD 482 μg/g). During the study period, 13 (18%) patients were hospitalized, four (5%) were admitted to an intensive care unit, and one patient died (Table 2). Except for the patient who eventually died, no-one needed mechanical ventilation. Among all hospitalized patients, the average number of days spent on a regular ward due to COVID-19 was 3.7 for CD, 6.3 for UC and 5.7 for all patients. Most patients (n = 62, 84%) were not on antibiotic therapy at the time of the COVID-19 infection. After the COVID-19 diagnosis had been established, 43% (n = 32) of all patients and 100% of those hospitalized received thrombosis prophylaxis.
Table 2: COVID-19-related symptoms, IBD activity and duration of hospitalization.
SD: standard deviation.
COVID-19 symptoms
The most common COVID-19 symptoms, presented in Table 2, were cough (56%), rhino-pharyngitis (51%), fever (46%), headache (34%), dyspnoea (26%), diarrhoea (24%), arthralgia/myalgia (16%), dysosmia/dysgeusia (15%), flu-like symptoms (13%), abdominal pain (12%) and nausea/vomiting (9%). Five percent did not develop any symptoms due to the COVID-19 infection. No thromboembolic complications were diagnosed.
Factors predicting hospitalization
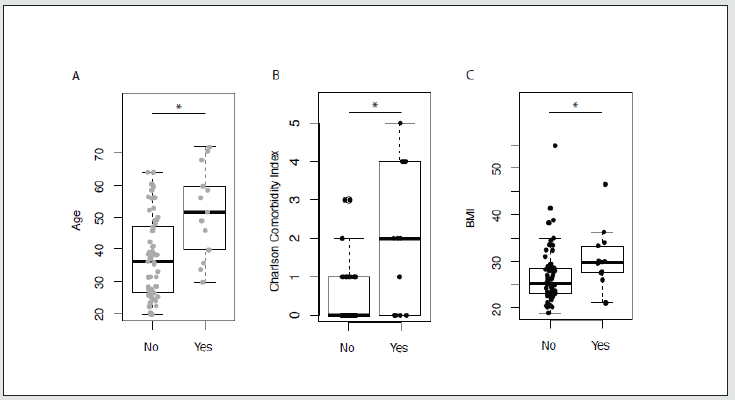
In all statistical analyses age, body mass index (BMI) and sex were examined simultaneously with the variable, and each variable was also examined alone. Increasing age was associated with a higher risk of hospitalization (p < 0.01), presented in (Figure 1A). With a CCI of two or more, the risk of hospitalization was significantly increased (p < 0.01 vs CCI 0–1), as seen in (Figure 1B). When the points for age were omitted from the CCI score, patients who had points due to an underlying medical condition were still more likely to be hospitalized (p < 0.01 vs no underlying medical condition).
Factors not predicting hospitalization
We could not demonstrate any significant association between BMI and risk of hospitalization, although there was a trend towards such a risk (p = 0.09). Interestingly, there was a significant association between BMI and hospitalization when one patient with a BMI of 54 was removed as an outlier (p = 0.02) (Figure 1C). Neither sex nor IBD type (UC or CD) had a significant impact on COVID-19 outcomes. There was no significant difference in hospitalizations among patients on biological medication, thiopurines or systemic corticosteroids, nor among patients who were taking any of the said medications as a combined therapy.
Figure 1: Factors predicting hospitalization. 1A) Age and risk of hospitalization. 1B) The Charlson Comorbidity Index (CCI) and risk of hospitalization. 1C) BMI and risk of hospitalization.
Patients (n = 17, 23% of all patients) who had biochemically active IBD (FC more than 200 μg/g during the past 6 months prior to COVID-19) were not hospitalized significantly more often than those with no preceding biochemical activity (n = 26, 35%); there was a non-significant tendency towards more frequent hospitalizations, but significance was not achieved due to the small number of patients. Neither previous clinical nor endoscopically active disease could be associated with hospitalization. Clinical, biochemical and endoscopic activity, as well as the results of patient records, were all examined at the same time for any indication of active disease prior to COVID-19, but there was still no significant change in hospitalization rates.
Discussion
Our study, which aimed at identifying risk factors for COVID-19 in IBD patients confirms previous findings indicating an association between hospitalization and both comorbidities and older age. Importantly, the use of any medication as maintenance therapy for IBD was not associated with an increased risk of a more severe COVID-19 infection or an undesirable outcome. The data of SECURE-IBD are likely to drive treatment recommendations for IBD during the COVID-19 pandemic. Immunosuppressive medications, especially thiopurines, used to treat IBD may result in a degree of immunosuppression, which has been hypothesized to lead to a more severe COVID-19 infection. Most of the patients in the IBD registry were on immunosuppressive treatment and were therefore thought to be at a higher risk of a severe COVID-19 infection. A recently published review article by Al-Ani and colleagues encourages the continuing of usual maintenance medications and highlights the importance of avoiding corticosteroids [20]. The finding of our study are in line with previous studies. In the case of an IBD flare-up, the risks and benefits of the treatments should be carefully discussed with the patient. More severe COVID-19 has been associated with older age and obesity [21-24]. Moreover, earlier studies have shown male sex to be a risk factor for more severe COVID-19 [25,26]. In the present study no significant association between sex and a higher risk of hospitalization was found. In the context of obesity, it is believed that the excess amount of adipose tissue causes inflammation and an impairment in the immune response. However, no significant correlation between BMI and the risk of hospitalization was found in our study. On the other hand, we found a significant association between age and hospitalization. It has been previously reported in studies of the general population that patients with comorbidities have a higher risk of more severe COVID-19 symptoms [27,28]. This was also seen in our population of IBD patients. Brenner and colleagues have found that increased age, comorbidities and, contrary to our study, also systemic corticosteroids are associated with severe COVID-19 in IBD patients [29].
Between 29 January 2020, when the first COVID-19 case in Finland was confirmed, and 15 April 2021, a total of 48,438 cases had been reported in the Hospital District of Helsinki and Uusimaa, which constitutes 2.8% of the population of 1.7 million [30]. In the present study, we identified 74 (1.4%) out of 5,194 patients in the IBD registry with a confirmed COVID-19 diagnosis since the beginning of the pandemic. The proportion of IBD patients with confirmed COVID-19 is less than half of the corresponding proportion of the general population of the same geographical area. Earlier studies have indicated that patients with IBD are at risk of serious opportunistic infections, particularly when they are treated with immunosuppressive medication. However, the findings of our study suggest that patients with IBD are not at a higher risk of contracting the SARS-COV-2 than the general population. This could be partly explained by a more rigorous hygiene routine. The patients in the Uusimaa Region have received detailed hygiene and health instructions from the specialized IBD nurses since the beginning of the pandemic. In the future, more data are needed on the social impact that the pandemic may have had on these immunocompromised patients. This study has some limitations. Firstly, the number of patients was limited as the incidence of COVID-19 in Finland has remained relatively low. Secondly, the lack of data in the patient records made it challenging to find variables associated with an unfavorable outcome and a higher risk of hospitalization. Additionally, during the pandemic, patients have tended not to attend scheduled laboratory follow-up tests, resulting in missing data. Despite these limitations, we believe that this study reflects well the real-life situation in clinical practice and provides important data on the COVID-19 infection in the IBD population.
The present study also has several strengths. Firstly, as the COVID-19 registry of the hospital district covers all reported cases in the region, it is extremely unlikely that a COVID-19-positive patient included in the IBD registry would have been missed in our search. Secondly, this study was performed in a country with a high IBD prevalence of one percent of the population [31] and with, consistent IBD treatment patterns and active patient organization counselling in place. All patients have equal access to treatment, and most hospital districts have specialized nurses who are trained to treat patients with IBD and advise them on travelling, vaccinations and hygiene. The observation period of this study mainly took place before the national vaccinations against COVID-19 started. Although no vaccine data is available for this study population, the number of COVID-19-vaccinated patients can be neglected, as IBD patients on immunosuppressive therapy were among the groups to receive the vaccine, starting from mid-April 2021. Some of the newest COVID-19 strains that have emerged after the data collection for this study have been found to be more infectious and linked to a higher mortality rate. Future studies will show whether the outcome of COVID-19 will differ from today’s studies.
Conclusion
This is the first report on the characteristics and outcomes of COVID-19 in patients with IBD in Finland, a country with a high prevalence of IBD and low prevalence of COVID-19. We found that comorbidities and older age were associated with a negative COVID-19 outcome such as hospitalization. On the other hand, immunosuppressive treatment for IBD was not associated with the risk of hospitalization or death. The lower incidence of COVID-19 infections among IBD patients in comparison to the general population may be explained by the rigorous hygiene measures undertaken particularly by patients on immunosuppressive therapy.
Author Contributions
Statement of authorship: study design (MK, PM, Ca B, PA), statistical analysis (JJ, JA), initial manuscript drafting (MK), critical revision and final approval (all authors).
Funding
The authors received no specific grant for this research. Patient involvement and patient consent for publication. Patients were not involved in the design, conduct reporting or dissemination plans of this research. Patient consent was not required.
References
- Alexander e. Gorbalenya, Susan C. baker, Ralph S. baric, Raoul J. de Groot, Christian Drosten, et al. (2020) Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. Nat. Microbiol 5: 536-544.
- Cucinotta D, Vanelli M (2020) WHO declares COVID-19 a pandemic. Acta Biomed 91(1): 157-160.
- Neurath MF (2020) COVID-19 and immunomodulation in IBD. Gut 69(7): 1335-1342.
- Seyed Hosseini E, Riahi Kashani N, Nikzad H, Azadbakht J, Hassani Bafrani H, et al. (2020) The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 551: 1-9.
- Chow N, Fleming-Dutra K, Gierke R, Hall A, Hughes M, et al. (2020) Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with COVID-19 - US, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 69(13): 382-386.
- Vardavas CI, Nikitara K (2020) COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis 18: 1-4.
- Lichtenstein GR, Loftus E V, Isaacs KL, Regueiro MD, Gerson LB, et al. (2018) ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol 113(4): 481-517.
- Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, et al. (2017) Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J Crohn’s Colitis 11(7): 769-784.
- Document DB (2018) FDA Briefing Document Gastrointestinal Drug Advisory Committee Meeting. DGIEP Briefing Document 1-71.
- Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, et al. (2019) AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 156(3): 748-764.
- Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, et al. (2014) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohn’s Colitis 8(6): 443-468.
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, et al. (2018) Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology 155(2): 337-346.e10.
- Long MD, Martin C, Sandler RS, Kappelman MD (2013) Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol 108(2): 240-248.
- Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, et al. (2021) Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 70(4): 725-732.
- Meyer A, Semenzato L, Zureik M, Weill A, Carbonnel F, et al. (2021) Risk of severe COVID-19 in patients treated with IBD medications: a French nationwide study. Aliment Pharmacol Ther 54(2): 160-166.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5): 373-383.
- Von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, et al. (2007) Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol 102(4): 803-813.
- Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, et al. (2008) Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 14(1): 40-46.
- Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, et al. (2020) Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev 14(6): 2103-2109.
- Al-Ani AH, Prentice RE, Rentsch CA, Johnson D, Ardalan Z, et al. (2020) Review article: prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment Pharmacol Ther 52(1): 54-72.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229): 1054-1062.
- Kassir R (2020) Risk of COVID-19 for patients with obesity. Obes Rev 21(6): e13034.
- Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, et al. (2020) High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 28(7): 1195-1199.
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, et al. (2020) Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA - J Am Med Assoc 323(16): 1574-1581.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, et al. (2020) Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 108: 154262.
- Zhu Z, Cai T, Fan L, Lou K, Hua X, et al. (2020) Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis 95: 332-339.
- Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, et al. (2020) Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 69(7): 1213-1217.
- Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, et al. (2020) Bowel Diseases: Results from an International Registry. Gastroenterology 159(2): 481-491.
- Finnish Institute for Health and Welfare. Situation update of coronavirus [Internet]. Infectious diseases and vaccinations.
- Entitlement: 2020 Prescription Register (including the purchases below the initial deductible) and Special Refund, Kela. R at. Finnish statistics on medicines 2019.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...