
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Review Article(ISSN: 2641-1652) 
The Mal-absorptive Canker-Enteropathy Associated T Cell Lymphoma Volume 4 - Issue 1
Anubha Bajaj*
- Consultant Histopathology, Punjab University, India
Received: November 07, 2022; Published: November 17, 2022
*Corresponding author: Anubha Bajaj, Consultant Histopathology, Punjab University, India
DOI: 10.32474/CTGH.2022.04.000180
Abstract
Enteropathy associated T cell lymphoma is an exceptionally discerned intestinal lymphoma derived from intraepithelial T cells commonly incriminating small intestine of subjects of celiac disease. Enteropathy associated T cell lymphoma occurring within gastrointestinal tract frequently delineates enteropathic alterations and manifests cytotoxic granule associated proteins. Additionally designated as intestinal T cell lymphoma, enteropathy associated T cell lymphoma type I, enteropathy type T cell lymphoma or malignant histiocytosis of the intestine, enteropathy associated T cell lymphoma comprises < 1% of non-Hodgkin’s lymphomas. Cogent staging necessitates bone marrow examination and computerized tomography (CT) of thorax, head and neck. Generally, lymphoma represents advanced stage disease. Lymphoma commonly arises within middle aged subjects or between sixth decade to seventh decade. An equivalent gender distribution is observed although a female predominance may ensue. Geographic distribution is variable with frequent incrimination of Caucasian population.
Enteropathy; T cell lymphoma; T cell receptor
Introduction
Enteropathy associated T cell lymphoma commonly arises within small intestine wherein jejunum, ileum or duodenum are involved in decreasing order of frequency. Metastasis into regional lymph nodes as mesenteric, para-aortic or iliac nodes may ensue. The occurrence of lymphoma within large intestine as isolated lesions or concurrent with small intestinal tumefaction are exceptionally discerned. Gastric region, spleen and hepatic or pulmonary parenchyma infrequently (<10%) demonstrate the neoplasm. Bone marrow is seldom involved. Enteropathy associated T cell lymphoma manifests various chromosomal gains and losses wherein gain of chromosome 9q33-34 is frequently observed. Enteropathy associated with T cell lymphoma exhibits clonal rearrangement of T cell receptor (TCR) gene. Array comparative genomic hybridization (aCGH) exhibits ~gain in chromosomes 1q32, 5q35, 7q22 or 8q24 ~loss of chromosome 8p22-p23, 9p21, 13q22 or 18q22. Loss of heterozygosity within chromosome 9p21 may be observed along with loss of p16 expression. Amplification of chromosome 9q33-34 is delineated. Besides, allelic imbalance of NOTCH1 or ABL1 gene and allelic variant of MYO9B is encountered. Genomic mutations within JAK/STAT and RAS pathways are enunciated. Occurrence of celiac disease is associated with enhanced possible emergence of non-Hodgkin’s lymphoma wherein majority (~65%) lymphomas concurrent with celiac disease delineate a T cell lineage. Gluten free diet exemplifies significant protection against development of lymphoma. However, delayed detection of celiac disease may appear concordant with progression of lymphoma.
Enteropathy associated T cell lymphoma (EATL) demonstrates an intense concurrence with refractory celiac disease. A variant of celiac disease, refractory celiac disease appears unresponsive to minimally 12 months of gluten free diet, upon morphological assessment. Besides, chronic intestinal inflammation engenders an aberrant population of intraepithelial T cells. Cogent detection of celiac disease and emergence of enteropathy associated T cell lymphoma demonstrates a variable duration of up to 10 years. Enteropathy associated T cell lymphoma manifests an aggressive clinical course. Cogent gastrointestinal symptoms as abdominal pain, diarrhoea, vomiting or malabsorption may occur. An estimated 50% of instances demonstrate complications such as gastrointestinal haemorrhage, perforation or obstruction. Discernment of celiac disease may concur with detection of enteropathy associated T cell lymphoma. Besides, B symptoms as fever, >10% loss of weight, night sweats, anorexia, fatigue, infection or dermatitis herpetiformis may arise. Hepatosplenomegaly and pruritus are exceptionally discerned. The majority (~75%) of instances delineate enteropathy within adjacent mucosa. Few lymphomas (~5%) enunciate a preceding diagnosis of celiac disease. Lymphoma commonly incriminates jejunum or ileum and is exceptionally discerned within various sites of gastrointestinal tract. Configuration of an ulcerated tumefaction with subsequent gastrointestinal obstruction or perforation may ensue. Tumour reoccurrence within intestinal region, mesenteric lymph nodes, hepatic or pulmonary parenchyma, spleen, bone marrow or cutaneous zones may ensue.
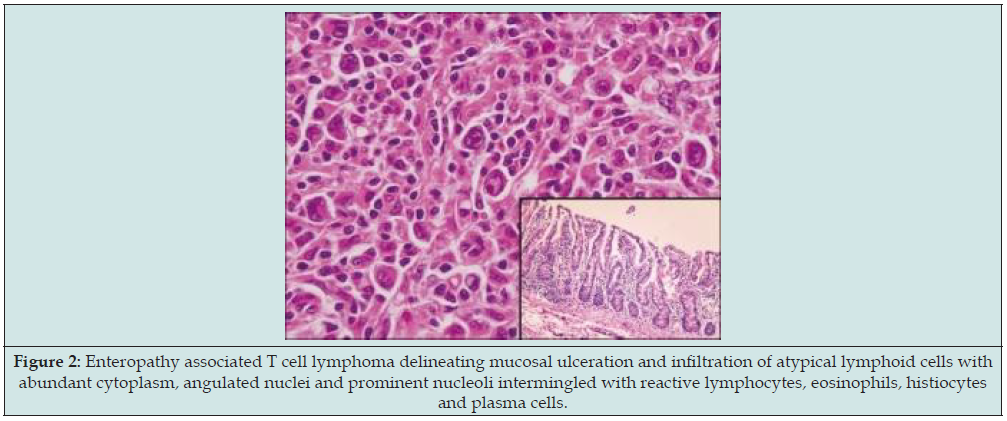
Enteropathy associated with T cell lymphoma commonly arises within adults. Occasionally, lymphoma may ensue following paediatric celiac disease. Prognostic outcomes are inferior. Grossly, small intestine exhibits a singular, enlarged mucosal ulcer or multiple, ulcerated lesions. Transmural occurrence of an enlarged tumefaction is infrequent. Besides, enlargement of regional mesenteric lymph nodes may ensue. Upon microscopy, incriminated intestine enunciates mucosal ulceration along with a diffuse, pleomorphic infiltrate of medium to enlarged atypical lymphoid cells incorporated with abundant cytoplasm, angulated nuclei and prominent nucleoli. The proportion of anaplastic cells is variable. A reactive inflammatory cell infiltrate composed of eosinophils, plasma cells, histiocytes and small lymphocytes appears admixed with neoplastic lymphocytes. Foci of necrosis are commonly discerned. Mitotic activity is significant. Angiotropism may ensue. Adjacent mucosa may enunciate features of celiac disease with elevated intraepithelial lymphocytes (> 30/100 enterocytes), focal crypt hyperplasia and villous atrophy. Incriminated mesenteric or regional lymph nodes exhibit partially effaced architecture with a preponderance of paracortical or sinusoidal pattern of neoplastic infiltration [1,2]. The ulcerated intestinal lymphoma demonstrates divergent histological manifestations wherein neoplastic cytotoxic T cells occur as regular, miniature, medium or enlarged, atypical, pleomorphic lymphoid cells. Cellular infiltration is commonly admixed with inflammatory cells as eosinophils and histiocytes. Frequently, enteropathic alterations confined to adjacent epithelium are discerned. The majority (~75%) of instances enunciate villous atrophy with crypt hyperplasia and disseminated intraepithelial lymphocytes. Lamina propria is inundated with lymphoid and plasmocytic infiltrate (Figures 1&2). Intestinal lymphoma staged with Lugano classification is denominated as
Figure 1: Enteropathy associated T cell lymphoma demonstrating infiltration of atypical lymphocytes with abundant cytoplasm, angulated nuclei and prominent nucleoli mixed with numerous congested blood vessels and focal villous atrophy.
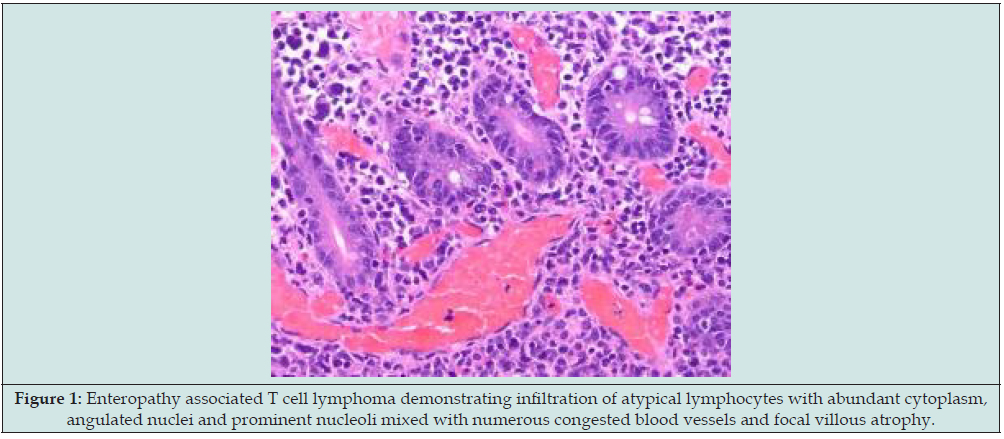
Figure 2: Enteropathy associated T cell lymphoma delineating mucosal ulceration and infiltration of atypical lymphoid cells with abundant cytoplasm, angulated nuclei and prominent nucleoli intermingled with reactive lymphocytes, eosinophils, histiocytes and plasma cells.
a) Limited stage disease comprised of
Stage I: Tumour involving singular lymph node or group of adjacent lymph nodes ~stage IE: Tumour involving singular extra-lymphatic site in the absence of regional lymph node involvement.
Stage II: Tumour involving ≥ 2 lymph node groups confined to one side of diaphragm ~stage IIE: Tumour demonstrating contiguous extra-lymphatic extension from a lymph node site along with or devoid of involvement of diverse lymph node regions on one side of diaphragm.
b) Advanced stage disease is constituted of
Stage III: Tumour incriminates regional lymph nodes on opposite sides of diaphragm or regional lymph nodes within supra-diaphragmatic area with involvement of spleen ~stage III.
(1): Tumour involves spleen or splenic, hilar, celiac or portal lymph nodes ~stage III.
(2): Tumour involves para-aortic, iliac, inguinal or mesenteric lymph nodes.
Stage IV: Tumour exhibits diffuse or disseminated involvement of ≥ one extra-nodal organ or tissue along with or devoid of regional lymph node involvement.
The lymphoma may indicate absence of systemic symptoms designated as ‘A’ symptoms or presence of systemic symptoms as pyrexia, night sweats or unexplained weight loss >10% body weight, denominated as ‘B’ symptoms. Extra-nodal contiguous extension of tumour mass which may be encompassed within an irradiation field appropriate for regional lymph node disease of identical anatomic extent is designated as ‘E’. Besides, extensive tumour expanse may be labelled as stage IV. ‘Bulky’ lymphoma is constituted of singular lymph node tumefaction >10centimetre magnitude or extending beyond >1/3rd of transthoracic diameter at any level of thoracic vertebrae as determined by computerized tomography (CT). Follicular non-Hodgkin’s lymphoma is categorized as bulky disease with tumour magnitude > 6 centimeters wherein bulky large B cell non-Hodgkin’s lymphoma exhibits a magnitude ranging from 6 centimeters to 10 centimeters. Staging of non-Hodgkin’s lymphoma necessitates evaluation of tumour diameter along long axis of incriminated lymph node or organ.
Enteropathy associated T cell lymphoma manifests T cell lineage markers as CD3, CD7, CD8, CD30, CD103, epithelial membrane antigen (EMA) and cytotoxic protein markers as T cell intracellular antigen 1(TIA1), perforin or granzyme B. Overexpression of p53 is observed. Few lymphomas are immune reactive to CD56 wherein majority (80%) of CD69+ instances express CD8. Occasional reactivity to CD79a is encountered. Lymphoma is immune nonreactive to CD4, CD5, CD7, CD20, CD56 or anaplastic lymphoma kinase (ALK). Enteropathy associated T cell lymphoma exhibits loss of T cell markers as CD2, CD4, CD5 or CD8. Besides, lymphoma expresses variable reactivity to Epstein Barr virus encoding RNAs (EBER). Clonal rearrangements of T cell receptor as TCR-β, TCR-γ and TCR- δ is delineated. Aforesaid clonal rearrangements may be discerned within adjacent mucosa. Refractory sprue may delineate clonal rearrangements of T cell receptor, thereby qualifying as a precursor lesion. Enteropathy associated T cell lymphoma requires segregation from neoplasms such as monomorphic epitheliotropic intestinal T cell lymphoma, indolent T cell lymphoproliferative disorder of gastrointestinal tract, peripheral T cell lymphoma not otherwise specified (NOS), extra-nodal NK/T cell lymphoma nasal type, adult T cell leukaemia/lymphoma, anaplastic lymphoma kinase (ALK) negative anaplastic large cell lymphoma, T lymphoblastic lymphoma, extra-nodal marginal zone lymphoma or ulcerative jejunoileitis. Enteropathy associated with T cell lymphoma can be appropriately detected with precise surgical tissue sampling.
Hematological assessment demonstrates microcytic anaemia and abnormal leukocyte count. Peripheral blood examination demonstrates antigliadin, anti-transglutaminase or anti endomysium IgA or IgG antibodies. Human leukocyte antigen (HLA) genotyping demonstrates the presence of HLA-DQ2, HLA-DQ5 or HLA-DQ8. Enteropathy associated T cell lymphoma can be assessed with 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) which is adopted for detecting long standing celiac disease, delineating a standard uptake value > 6. Upon computerized tomography (CT), features such as thickening of bowel wall, cavitation of mesenteric lymph nodes, intestinal intussusception or miniature spleen (< 120 cm3) are exemplified. Video capsule endoscopy is optimal in determining extent and precise location of lesion or gastrointestinal haemorrhage. Enteropathy associated T cell lymphoma can be subjected to surgical extermination, debulking and resection of tumefaction. Surgical intervention is optimally adopted in lymphomas demonstrating complications such as gastrointestinal obstruction or perforation. Adoption of standard dose combination chemotherapy exemplifies complete remission in < 50% subjects. Commonly employed chemotherapeutic regimens are cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) or CHOP-like regimen. Subjects unresponsive to first line CHOP therapy exhibit inferior prognostic outcomes. Besides, combinations such as bleomycin, doxorubicin, cyclophosphamide, vincristine and prednisone (BACOP) or vincristine, doxorubicin, high dose methotrexate and prednisolone (VAMP) or prednisolone, doxorubicin, cyclophosphamide, etoposide, mechlorethamine, vincristine and procarbazine (ProMACE-MOPP) or cisplatin, cytarabine, etoposide and methylprednisolone (ESHAP) or carmustine, etoposide, cytarabine and melphalan (BEAM) can be beneficially adopted.
Conclusion
Combination of high dose chemotherapy followed by therapeutic consolidation with autologous stem cell transplantation is advantageous and exemplifies long term disease remission in eligible subjects. Employment of radiation therapy is debatable. Novel therapeutic agents such as alemtuzumab, cladribine, romidepsin can be advantageously utilized. Enteropathy associated T cell lymphoma enunciates an adverse prognostic outcome with median overall survival at ~10 months and 5-year overall survival at ~ 20%. Prediction of survival may be obtained with prognostic index for peripheral T cell lymphoma (PIT) score wherein parameters such as lactate dehydrogenase (LDH), age of incriminated subject, bone marrow involvement and performance status can be evaluated. Factors enunciating superior overall survival are designated as ~serum albumin levels > 21.6 grams/ Litre ~commencement of chemotherapy ~adoption of surgical resection. Factors contributing to inferior overall survival are denominated as ~enlarged tumour mass > 5centimetre magnitude ~non-ambulatory performance status ~elevated values of serum lactate dehydrogenase (LDH) and C reactive protein (CRP) [3,4].
References
- Chen M, Liu X, Zhang Y, Shi Y (2022) Endoscopic features and clinical outcomes of enteropathy-associated T-cell lymphoma: A tertiary center retrospective study. Saudi J Gastroenterol 28(2): 127-134.
- Mashayekhi A, Quiroga EF Margolick JF, Post GR (2022) Intestinal T-cell lymphoma: A rare entity presenting with severe acute upper quadrant pain. Clin Case Rep 10(3): e05546.
- Yeh SP, Lin HL, Chien CR (2022) Long term survival of Enteropathy-associated T-cell Lymphoma (EATL) with intracranial metastasis: letter to the Editor. Acta Gastroenterol Belg 85(3): 535-536.
- Kronsten VT, Gosson C, Khatib AA, Bagwan I, Worthington T, et al. (2022) Tonsillar Extraintestinal Enteropathy-Associated T-Cell Lymphoma in a Patient with Celiac Disease. ACG Case Rep J 9(7): e00824.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


