Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
The Characteristics and Clinical Implication of the Resting- State Brain Function Assessed by BOLD-fMRI in Patients with Active Gastroesophageal Reflux Disease Volume 4 - Issue 3
Liwei Dong1, Deyong Liu2, Chaochao Chen3, Yingman Zhao1, Fei Wang1, Ming Li1 and Cheng Lan3*
- 1Department of Radiology, Hainan General Hospital, Affiliated Hainan Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China
- 2Department of Gastroenterology, Hunan University of Environment and Biology, Hengyang, Hunan, People’s Republic of China
- 3Department of Gastroenterology, Hainan General Hospital, Affiliated Hainan Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China
Received: June 02, 2023; Published: June 07, 2023
*Corresponding author: Cheng Lan, Department of Gastroenterology, Hainan General Hospital, Affiliated Hainan Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China
DOI: 10.32474/CTGH.2023.04.000186
Abstract
Objective: The current study’s objective is to investigate the characteristic and clinical implication of resting-state brain function assessed by BOLD-fMRI in patients with active gastroesophageal reflux disease.
Methods: 31 GERD patients were scanned by BOLD-fMRI before and after the standard anti-reflux treatment. The characteristics of the fMRI image and signal were analyzed. The scanning data including regional homogeneity (ReHo), amplitude of low frequency fluctuation (ALFF), functional connectivity (FC) was calculated. The relationship of the active region and the clinical symptoms was analyzed by spearman correlation analysis software.
Results
a) The GERD patients in active stage show abnormally active in some functional brain regions,resuming after treatment.
b) ReHo and ALFF significantly decreased after treatment (P<0.05), but unchanged in FC (P>0.05).
c) considering the sex, age, and education, ReHo significantly increased (P<0.05), but no changes in FC (P>0.05).
d) the changes of the fMRI in GERD patients were in accordance with the changes of their clinical score (r=0.390, p<0.05).
Conclusion: our results suggested that BOLD-fMRI could find the characteristic active region in GERD patients, whose brain functional changes could be related to the active stage and their sex, age and education.
Gastroesophageal reflux disease; MRI; resting state; brain functional imaging; mechanism
Introduction
Gastro-oesophageal reflux disease (GERD) is a series of symptoms resulting from the refluxing of the gastric contents into the esophageal. A significant lesion in the esophageal was not found in most patients under endoscopy [1,2]. The diagnosis of this disease is relatively difficult, and the patients suffer from refractory discomfort and high medical expenses [3,4]. Due to the unclear pathogenesis, effective treatment remains to be found [5]. Accompanied with the development of the studies on visceral sensory nerves,neuroelectrophysiology and functional brain imaging, the underlying mechanism of GERD becomes more and more clear. A lot of research focused on the esophageal visceral hypersensitivity induced by the local peptidergic nerves, the peripheral nociceptive receptors sensitization and the abnormal pathway from esophageal to the central including the active dorsal horn of spinal cord, neurons sensitization and neuroplasticity change [6-8]. During the last two decades, non-invasive brain functional imaging and cortical evoked potential techniques make it possible to deeply investigate the complicated mechanism of visceral hypersensitivity associated diseases [9-11].
Positron emission tomography (PET) and functional magnetic resonance imaging(fMRI) technique were used to explore the central pathway of the visceral hypersensitivity [12,13]. fMRI is a kind of MRI based on the magnetic sensitivity effect of the deoxyhemoglobin, combining nerve activation and MRI with high resolution into brain image, with high spatial resolution and no need of radionuclide [14,15]. Currently, fMRI is applied to study the irritable bowel syndrome but not GERD [16-19] scanned the healthy person and GERD patients stimulated by intra-esophageal acid with fMRI and explored the relationship between the heartburn symptom and the brain function. Acid stimulation could mimic GERD but could not reveal the real process during different stages. Thus, current research is to instigate the characteristics and clinical implication of the resting-state brain function assessed by BOLD-fMRI in patients with active GERD.
Material and Methods
Objectives
31 GERD patients diagnosed according to the standard from the outpatient and inpatient department were enrolled [20], including 14 male and 17 female, with the average age of 35. The patients were treated with the standard anti-reflux protocol (lansoprazole+mosapride+hydrotalcite, 8 weeks). GERD-Q scales were used before and after the treatment [21]. The informed consent was signed by the patients and the research protocol was approved by the Ethics Committee of Hainan General Hospital.
MRI scanning
Instrument
The patients were scanned by Siemens Prisma 3.0T MRI, with Ultra-high-speed inversion gradient additional magnetic field coil for echo plane photography for signal position coding and image reconstruction and processing software. The standard 20 chanel head-neck phased array coil was used during the scanning.
Scanning parameters
Firstly, the patients’ whole brain construction in sagittal position T1 was scanned with MPRAGE sequence. The total 192 level covered the whole brain from the overhead to the inferior margin of the cerebellum. T1-MPRAGE scanning parameter was repeated time (TR)/Echo time (TE) =1750/2.31, Matrix 256X256, NEX=I. Single shot gradient echo planar imaging sequence and BOLD imaging mode were used to enhance the signal contrast ratio. During the imaging, Tl WI-MPRAGE worked as the anatomic background. The functional image was stacked in real time on the anatomic image and the result was evaluated. The parameter of the EPI functional imaging was as follows: Vision FOV: 192mmX192 mm, the resolution was 2mm per pixel. Thickness of fault/Fault intervals=2.1mm/0.525mm, pulse repeated intervals time/Echo time (TR/TE) = 3000/30 ms, Flip angle: 90’, the scanning time: 3 minutes and 11seconds (the total scanning time was about 7 minutes. All the scanning data was collected by one technician in MRI room, Department of Radiology, Hainan General Hospital.
Image analysis and data post-processing
Two MRI doctors evaluated the images with no idea of the patients’ clinical information. All data was analyzed by the software within the scanning system. The first two image data was abandoned to avoid the possible artifact and magnetic saturation induced by the hemodynamics changes. The region of interest (ROI) was established, with which the data of the respective exciting brain region was t-tested. The threshold was 2500-3000. The differential images reconstructed with matching subtraction were integrated with the structural image and the signal characteristics in the cortex exciting region were analyzed. The voxel of the individual data in every group was calculated one by one. The exciting status was confirmed with a t-test between the voxel and its own basic status according to the different mission directed BOLD response. The significance value was P<0.01. More than 5 connected pixels were considered as an activated region. The exciting region in which the signal ascended was an exciting brain region presented by white color. The exciting status of the visceral sensory centers was assessed with the max mean signal intensity and changing range of the ROI.
The structural and functional image was processed (matlab+spm12+RESTPlus). Slice timing made every scanning result tend to be close to the real-time result. Realign modulated the brain position at every time point and confirmed that the data direction of all the time point keep consistent to minimize the noise from the head moving during the scanning period. The head moving data was used as the evaluation standard of the image quality. Normalize means that the space was standardized. Two tested T1 images were configurated to the mean image of its own BOLD functional one. The changed structural image was dissected into gray matter, white matter, and cerebrospinal fluid. The gray matter image was configured to the organization probability map. The nonlinear transformation parameters were utilized to the head-moving corrected volume, obtaining the functional image in the MNI standard space. The basic data included regional homogeneity (ReHo) , amplitude of low frequency fluctuation (ALFF) and functional connectivity (FC).
Statistical analysis
All data were analyzed using Grubbs’ test and then performed by using the one-way analysis of variance (ANOVA) to test homogeneity of variances via Levene’s test and followed with Ducan’s multiple comparison test (SPSS 22.0 software). Data are expressed as the mean ± standard error of the mean. Values in the same row with different superscripts are significant (P<0.05), while values with the same superscripts are not significantly different (P>0.05). P<0.05 was a statistically significant difference. Spearman correlation analysis was used to investigate the correlation between the image data and the clinical symptoms. Correlation value: >0.8, strong correlation, 0.3-0.8, weak correlation, <0.3, no correlation.
Results
The characteristic change of the brain functional image in GERD patients
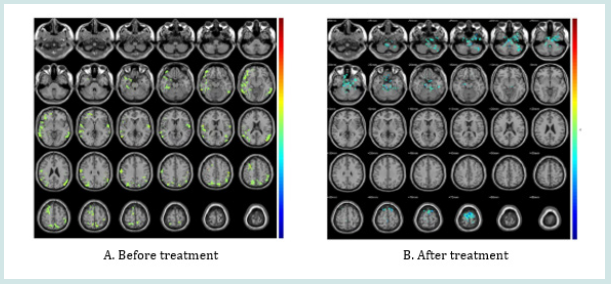
The brain regions in GERD patients significantly show activation, including Temporal_Inf_L/R, Fusiform_L, Paracentrol_ Lobule_L, Postcentrol_R, Precentrol_R, Frontal_Sup_L, Occipital_ Mid_R, Parietal_Inf-L. After treatment, most of the activated region resumed to rest status (Figure1).
ReHo
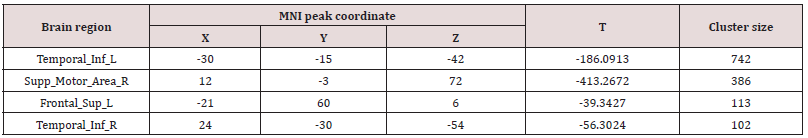
After treatment, the ReHo value of the Temporal_Inf_R, Supp_ Motor_Area_R and Frontal_Sup_L significantly decreased (p<0.05, Table 1).
ALFF
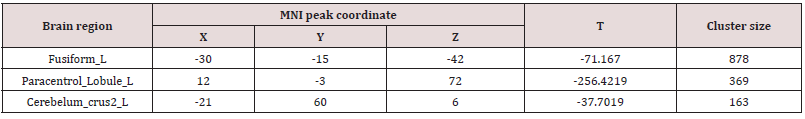
After treatment, the ALFF value of the Fusiform_L, Paracentrol_ Lobule_L and Cerebelum_crus2_L significantly decreased (p<0.05, Table 2).
FC
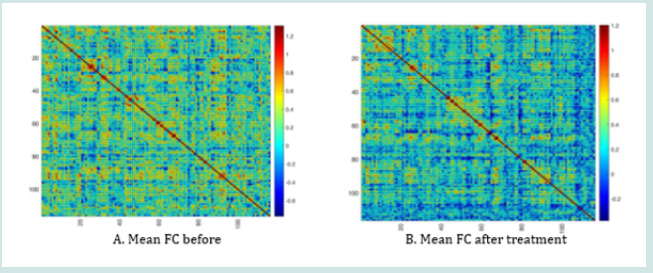
After treatment, the FC value of the GERD patients did not significantly change (p>0.05, Figure 2).
The impact of gender, age, and education degree on the brain function in GERD patients
After corrected with gender, age, and education degree, the ReHo value of Postcentrol_R, Occipital_Mid_R, Parietal_Inf-L, Precentrol_R, Frontal_Mid_Orb_R, Frontal_Inf_Orb_R, Temporal_ Mid_L and Temporal_Sup_L significantly decreased (p<0.05, Table 3).
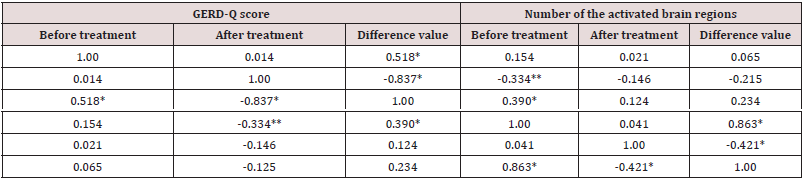
The correlation between the number of the activated brain regions and the clinical symptoms scores in GERD patients
The GERD-Q score after treatment was negative correlated with the number of the activated brain regions, r=-0.334, but no significant changes, p>0.05 (Table 4). The difference value of GERD-Q score before and after treatment was positive correlated with the number of the activated brain regions, r=0.390, significant, p<0.05 (Table 4). These results suggested that the more active the brain functions, the more effective was the treatment.
Table 4: The correlation between the number of the activated brain regions and the clinical symptoms scores in GERD patients.
Discussion
BOLD-fMRI comprises resting-state and mission-state, revealing the neuro-pathological mechanism and supplying a lot of image information. Compared with mission-state, restingstate BOLD-fMRI is easily available, with high repeatability and independent from pecial mandatory stimuli. It was observed that in the active GERD patients, the brain function regions were significantly excited, including Temporal_Inf_L/R, Fusiform_L, Paracentrol_Lobule_L,Postcentrol_R, Precentrol_R, Frontal_Sup_L, Occipital_Mid_R, Parietal_Inf-L. After treatment, most of the activated region resumed resting status. Temporal_Inf_L/R helps to learn three-dimensional objects. Fusiform_L helps to distinguish the secondary classification of objects. Paracentrol_Lobule_L is involved in the motor and sensory of the lower part of body. Postcentrol and Precentrol were the motor and sensory centers, associated with general anxiety disorder, Frontal_Sup_L is related with depression. Occipital_Mid_R, and Parietal_Inf-L help cognitive function [22]. These brain functions could be involved in visceral hypersensitivity. The excited brain function became resting state after anti-reflux treatment, suggesting that the brain function region’s activation could participate in the pathogenesis of GERD. In addition, the difference in value of GERD-Q score before and after treatment was positively correlated with the number of the activated brain regions, suggesting that the more active were the brain function, the more effective was the treatment.
Thus, the assessment of brain function could be a potential parameter in the evaluation of the therapy efficiency in GERD. ReHo reflects the difference of the act in local and whole brain region, helping to investigate the neurons act homogeneity in resting state and find the neural circuit between the functional and anatomic structure [23]. We found that the ReHo the value of Temporal_Inf_R, Supp_Motor_Area_R and Frontal_Sup_L significantly increased and after treatment significantly decreased. These regions were mainly involved in cognition and depression [24], suggesting that during active GERD, the brain cognition function could play an important role. Corrected with gender, age, and education degree, the ReHo value decreased after treatment, suggesting that in the diagnosis and treatment of GERD, the patients’ individual information should be considered, which could be associated to their brain function [25]. ALFF indicates the brain neurons’ spontaneous act through detect the BOLD signal’s changing amplitude relative to the baseline and is usually used to explore schizophrenia and Parkinson’s disease [26,27]. The ALFF value of the Fusiform_L, Paracentrol_Lobule_L increased before treatment and decreased after treatment.
Fusiform_L is responsible for the cognition of secondary classification of objects, and Paracentrol_Lobule_L participates in the motor and sense of the lower body. These abnormally activated brain functional regions could be the underlying pathogenesis of GERD. Wagner et al. reported that depression patients with suicide tendency show decreased ALFF value in their frontoparietal network and increased ALFF value in hippocampus and thalamus. Raikle et al. [28] reported that there exists brain default network in resting state, including partially activated cingulate gyrus, central prefrontal cortex, and bilateral temporal parietal lobe area, which was related to cognition and emotion. We did not observe this network in the current research. The heterogeneity of GERD may be one of the possible factors. FC reflects the collaboration among the individual brain functional regions through analyzing the time correlation between the brain function network and the individual brain functional regions [29]. FC value increased in some brain regions in patients with insomnia, prompting the role of brain function region’s collection in the somnipathy [30]. However, the FC value of the GERD patients did not significantly change after treatment, suggesting that more precise mechanisms, for example, the forward/positive and reverse/negative feedback loop remains to be intensively studied.
Conclusion
The current study’s shortcoming is as follows: we did not monitor the patients with electroencephalogram and could not confirm whether the patients were in a real resting state. On the other hand, the material basis of the changes in the brain function regions remains to be explored. In one word, resting-state BOLDfMRI could find the characteristic active function region in GERD patients, whose brain functional changes could be related to the active stage and their gender, age, and education.
Funding
The current research was supported by Natural Science Foundation of China (No. 82060102), Natural Science Foundation of Hainan Province, High-level Personnel Program (No. 822RC818), Research Project of Health Industry in Hainan Province(No. 20A200172), and Hainan Provincial Clinical Medical Center.
References
- Young A, Kumar MA, Thota PN (2020) GERD: A practical approach. Cleve Clin J Med 87(4): 223-230.
- Chen J, Brady P (2019) Gastroesophageal Reflux Disease: Pathophysiology, Diagnosis, and Treatment. Gastroenterol Nurs 42(1): 20-28.
- Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, et al. (2017) Review Team: World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol 51(6): 467-478.
- Katzka DA, Kahrilas PJ (2020) Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ 371: m3786.
- Sharma P, Yadlapati R (2021) Pathophysiology and treatment options for gastroesophageal reflux disease: looking beyond acid. Ann N Y Acad Sci 1486(1): 3-14.
- Hobson AR, Sarkar S, Furlong PL, Thompson DG, Aziz O (2000) Identification of the optimal parameters for recording cortical potentials evoked by mechanical stimulation of the human esophagus. Neurogastroenterol Motil 12(2): 163-171.
- Zheng Z, Shang Y, Wang N, Liu X, Xin C, et al. (2021) Current Advancement on the Dynamic Mechanism of Gastroesophageal Reflux Disease. Int J Biol Sci 17(15): 4154-4164.
- Aggarwal P, Kamal AN (2020) Reflux Hypersensitivity: How to Approach Diagnosis and Management. Curr Gastroenterol Rep 22(9): 42.
- Ribeiro M, Forcelini CM, Navarini D, Soder RB, Fornari F (2022) Disruption of the brain-esophagus axis in obese patients with heartburn. Dis Esophagus 35(11): doac021.
- Xu S, Zheng F, Zhao X, Chen Y, Kong X, et al. (2010) Brain processing of visceral sensation upon esophageal chemical stimulation in different types of GERD. Eur J Radiol 75(3): 352-359.
- Hollerbach S, Bulat R, May A, Kamath MV, Upton AR, et al. (2000) Abnormal cerebral processing of oesophageal stimuli in patients with noncardiac chest pain (NCCP). Neurogastroenterol Motil 12(6): 555-565.
- Wu YW, Tseng PH, Lee YC, Wang SY, Chiu HM, et al. (2014) Association of esophageal inflammation, obesity and gastroesophageal reflux disease: from FDG PET/CT perspective. PLoS One 9(3): e92001.
- Wang K, Duan LP, Zeng XZ, Liu JY, Xu-Chu W (2011) Differences in cerebral response to esophageal acid stimuli and psychological anticipation in GERD subtypes--an fMRI study. BMC Gastroenterol 11: 28.
- Bollmann S, Barth M (2021) New acquisition techniques and their prospects for the achievable resolution of fMRI. Prog Neurobiol 207: 101936.
- Gonzalez-Castillo J, Kam JWY, Hoy CW, Bandettini PA (2021) How to Interpret Resting-State fMRI: Ask Your Participants. J Neurosci 41(6): 1130-1141.
- Sarnoff RP, Bhatt RR, Osadchiy V, Dong T, Labus JS, et al. (2023) A multi-omic brain gut microbiome signature differs between IBS subjects with different bowel habits. Neuropharmacology 225: 109381.
- Yu Z, Liu LY, Lai YY, Tian ZL, Yang L, et al. (2022) Altered Resting Brain Functions in Patients with Irritable Bowel Syndrome: A Systematic Review. Front Hum Neurosci 16: 851586.
- Kern M, Hofmann C, Hyde J, Shaker R (2004) Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol 286(1): 174-181.
- Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, et al. (2018) Modern diagnosis of GERD: the Lyon Consensus. Gut 67(7): 1351-1362.
- Roark R, Sydor M, Chatila AT, Umar S, Guerra R, et al. (2020) Management of gastroesophageal reflux disease. Dis Mon 66(1): 100849.
- Humphreys GW, Price CJ (2001) Cognitive neuropsychology and functional brain imaging: implications for functional and anatomical models of cognition. Acta Psychol (Amst) 107(1-3): 119-153.
- Fang X, Zhang R, Bao C, Zhou M, Yan W, et al. (2021) Abnormal regional homogeneity (ReHo) and fractional amplitude of low frequency fluctuations (fALFF) in first-episode drug-naïve schizophrenia patients comorbid with depression. Brain Imaging Behav 15(5): 2627-2636.
- Zacková L, Jáni M, Brázdil M, Nikolova YS, Marečková K (2021) Cognitive impairment and depression: Meta-analysis of structural magnetic resonance imaging studies. Neuroimage Clin 32: 102830.
- Kamolz T, Bammer T, Pasiut M, Pointner R (2002) Psycho-physiological aspects of gastroesophageal reflux disease. Psychother Psychosom Med Psychol 52(3-4): 159-165.
- Zhao C, Zhu J, Liu X, Pu C, Lai Y, et al. (2018) Structural and functional brain abnormalities in schizophrenia: A cross-sectional study at different stages of the disease. Prog Neuropsychopharmacol Biol Psychiatry 83: 27-32.
- Yue Y, Jiang Y, Shen T, Pu J, Lai HY, et al. (2020) ALFF and ReHo Mapping Reveals Different Functional Patterns in Early- and Late-Onset Parkinson's Disease. Front Neurosci 14: 141.
- Gerd Wagner, Meng Li, Matthew D Sacchet, Stéphane Richard-Devantoy, Gustavo Turecki, et al. (2021) Functional network alterations differently associated with suicidal ideas and acts in depressed patients: an indirect support to the transition model. Transl Psychiatry 11(1): 100.
- Raichle ME (2015) The brain's default mode network. Annu Rev Neurosci 38: 433-447.
- Fingelkurts AA, Fingelkurts AA, Kähkönen S (2005) Functional connectivity in the brain--is it an elusive concept? Neurosci Biobehav Rev 28(8): 827-836.
- Fasiello E, Gorgoni M, Scarpelli S, Alfonsi V, Ferini Strambi L, et al. (2022) Functional connectivity changes in insomnia disorder: A systematic review. Sleep Med Rev 61: 101569.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...