Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
Randomized Control Study Evaluating Effect of Vegetable Protein Diet in Liver Cirrhosis with Hepatic Encephalopathy in a Tertiary Care Hospital in India Volume 4 - Issue 1
Akshay Rawat1*, Anurag Govil2, Dinesh Agrawal2, Harsh Udawat3 and Sandeep Vaishnav3
- 1Resident Doctor, Department of Gastroenterology, Santokba Durlabhji Memorial Hospital & Research Centre, India
- 2Senior consultant, Department of Gastroenterology, Santokba Durlabhji Memorial Hospital & Research Centre, India
- 3Consultant, Department of Gastroenterology, Santokba Durlabhji Memorial Hospital & Research Centre, India
Received: September 30, 2022; Published: October 11, 2022
*Corresponding author: Akshay Rawat, Resident Doctor, Department of Gastroenterology, Santokba Durlabhji Memorial Hospital & Research Centre, Jaipur, India
DOI: 10.32474/CTGH.2022.04.000177
Abstract
Background and Aims: Hepatic encephalopathy (HE) portend a worse survival for cirrhotic patients compared to similar patients without HE, even after accounting for the MELD score. Restriction of dietary protein intake in HE has been practiced, based on uncontrolled observations, which could predispose cirrhotic to malnutrition. This randomized study was conducted to evaluate the effects of optimal protein supplementation through daily diet in patients of liver cirrhosis with HE.
Methods: This study was conducted in a tertiary hospital, enrolling patients of cirrhosis with HE and randomizing them into two groups – Group A patients were given optimal protein diet (1.5 g/kg/day) while Group B patients continued to take their routine diet. Patients were re-evaluated at one month for improvement/worsening in HE and for improvement in their nutritional parameters and HRQOL.
Results: Patients who were given optimal protein supplementation had statistically significant improvement in serum albumin, hand grip, skeletal muscle mass and HRQOL along with a non-significant improvement in CTP score, MELD Na, Fasting arterial ammonia, PHES score, MAC and TSF.
Conclusion: This study highlights the potential benefits of providing adequate nutritional support to cirrhotic patients with HE. In fact, nutritional parameters like MAC, TSF and skeletal muscle mass decreased in patients who were on unsupervised suboptimal diet which could further lead to protein energy malnutrition.
Cirrhosis; malnutrition; hepatic encephalopathy; protein supplementation
Introduction
Cirrhosis is a diffuse hepatic fibrosis with the replacement of normal liver architecture by nodules. In addition, the complications of cirrhosis include, portal hypertension, ascites, hepatorenal syndrome, and hepatic encephalopathy (HE). Hepatic encephalopathy manifests as a wide spectrum of neurological or psychiatric abnormalities ranging from subclinical alterations to coma [1]. HE develops in 50% to 70% of patients with cirrhosis, and its occurrence is a poor prognostic indicator, with projected 1- and 3-year survival rates of 42% and 23%, respectively, without liver transplantation [2]. HE that is clinically apparent is termed as overt hepatic encephalopathy (OHE). HE that is mild, without an obvious clinical profile and diagnosed only by specialized cognitive testing is termed as covert hepatic encephalopathy (CHE), also known as minimal (MHE) or subclinical hepatic encephalopathy [3]. There is strong evidence that patients with advanced cirrhosis, have malnutrition [4] which is under diagnosed. Ammonia (NH3) is thought to be central in the pathogenesis of HE and the loss of muscle mass can adversely affect the ability of muscles to remove ammonia.
Malnourished cirrhotic patients have higher chances of infections and other complications requiring longer hospital stay v [5-8]. Protein restriction has been followed for patients with hepatic encephalopathy [9] as part of treatment however it lacks scientific evidence from literature studies [10]. The latest studies advocate against protein restriction for HE patients [11-13]. Now it is imperative that dietary, nutritional assessment of cirrhotic patients are done so that optimum protein intake can be supplemented to patients. The paucity of Indian studies makes present work more relevant as it would be helpful in assessing nutritional status which is often neglected [14]. The current study assessed the effects of vegetable protein supplementation given as recommended by The European Society for Clinical Nutrition and Metabolism [15].
Methods
The prospective study included all adult patients of liver cirrhosis with hepatic encephalopathy who were admitted in the department of gastroenterology at tertiary care centre in western India between October 2018 to March 2020. Liver cirrhosis was diagnosed on the basis of clinical features, laboratory tests, endoscopic findings, ultrasonography abdomen and liver biopsy. The study was approved by the institutional ethics committee and subjects were recruited after obtaining informed consent in writing.
The following were the exclusion criteria for the study participants:
a) non-adherence of patient to advised diet (average protein intake of < 80% of the prescribed protein/day in the intervention group for < 80% of days of the study).
b) alcohol intake in the past 6 weeks before enrolment or during the period of study.
c) suspected/diagnosed hepatocellular carcinoma.
d) history of trans jugular intrahepatic porto-systemic shunt (TIPS) recent (< 6 weeks).
e) history of gastrointestinal haemorrhage.
f) patient with neurological or psychiatric problems.
g) patients on psychotropic drugs.
h) poor vision precluding neuropsychiatric assessment.
i) creatinine >1.3 mg/dl in males or >1.1 mg/dl in females.
j) electrolyte imbalance (serum sodium <130 or >150 mmol/l, serum potassium <3.0 or >5.5 mmol/l).
k) Sepsis.
l) refractory ascites or tense ascites that required therapeutic peritoneocentesis during the study period or poor compliance to pharmacological therapy.
m) and irregular follow-up visits.
Patients were randomized into two groups (Group A – intervention group and Group B – control group). Patients in Group A (intervention group) were counselled by a dietician and prescribed a diet according to ESPEN guidelines [16] (30 - 35 kcal/ kg of ideal body weight/day: 1.5 g/kg of ideal body weight/day vegetable protein). Patients in the Group B (control group) were given normal routine diet. Patients with ascites were advised to restrict sodium intake to < 2g/day. The subjects were instructed to maintain daily diet chart and a regular follow up for adherence to instructions was made by weekly telephonic follow up. The target was to attain >80% of protein requirement. The primary end points were – readmission (with or without worsening HE) and improvement on nutrition parameters. The secondary end points were improved Health related quality of Life (HRQOL), Child- Turcotte-Pugh score and MELD score (Figure 1). The outcomes were measured initially at the time of recruitment and after end of study period (3 months) similar to study [17].
The sample size for study and control group was 30 each calculated at 80% study power and alpha error of 0.05 assuming standard deviation of 24.753 umol/l. The continuous variables were expressed as mean and standard deviation, they were compared using unpaired t test. Nominal variables were summarised as proportions and analyzed by chi square test while categorical variables by Fischer exact test. Ordinal variables were expressed as median and range, analyzed by Mann Whitney test. The correlation between continuous & ordinal variables measured by Pearson and spearman’s coefficient respectively. MHE was diagnosed using psychometric hepatic encephalopathy score (PHES) that using pscychometeric tests and those with score <5 was labelled as MHE [18] HRQOL was measured using SF-36 questionnaire [19].
Results
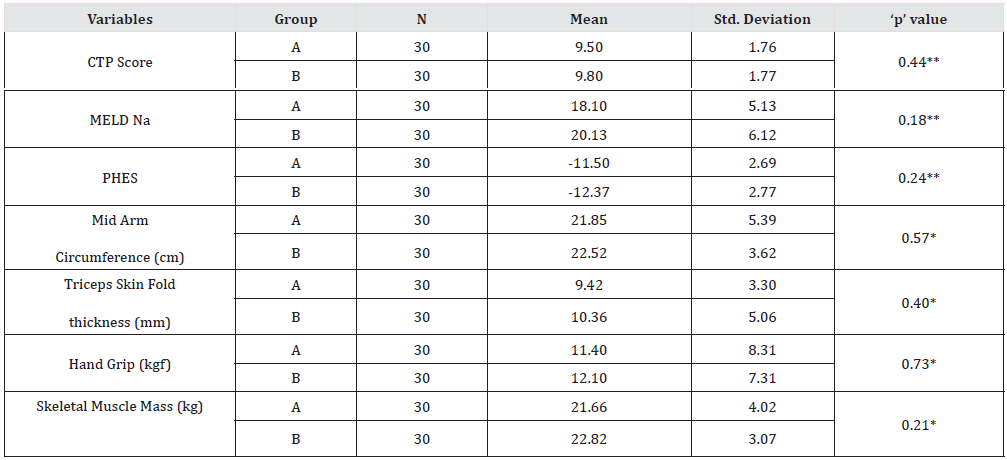
The prospective study was conducted in the department of gastroenterology in tertiary care hospital. A total of 86 patients were randomized into two groups of 41 each- group A (with dietary intervention) and Group B (Without dietary intervention). At the end of study period 30 patients were finally analyzed from each group. The mean age of study group was 53.43 years with M:F ratio 5:1 whereas control group the mean age was 51.03 years with M:F ratio 9:1. The differences were statistically insignificant (Table 1). The mean bilirubin, albumin, ammonia, and INR in the study group were 3.95 mg/dl, 2.79 g/dl, 101.60 umol/l and 1.77 respectively while for group B it was 5.37 mg/dl, 2.81 g/dl, 108.70 umol/L and 1.81. The difference was not statistically significant (Table 2).
* Unpaired ‘T’ Test, ** Mann-Whitney Rank Sum Test, *** Fisher Exact Test.
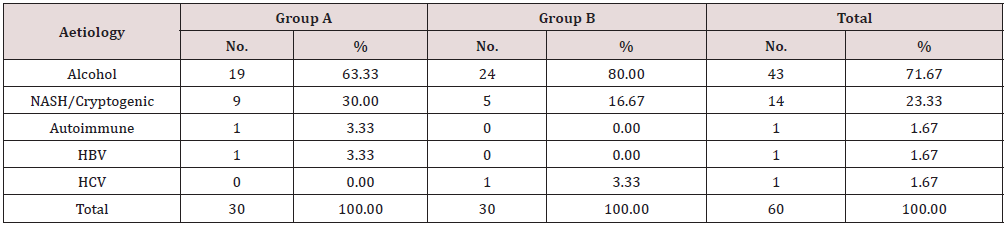
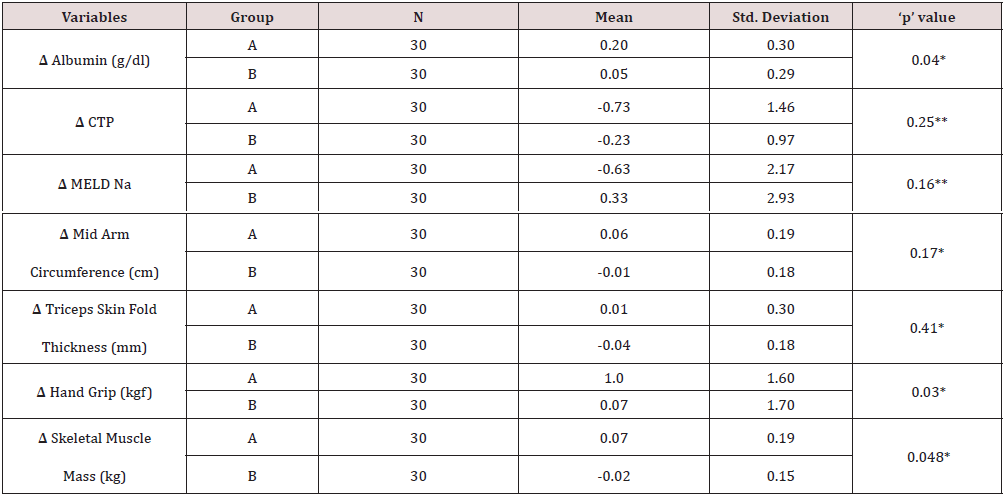
The nutritional assessment parameters for group A included CTP score, MELDNa, PHES, MAC, TSFT, Handgrip and skeletal muscle mass were 9.50, 18.10, -11.50, 21.85 cm, 9.42 mm, 11.40 kg and 21.66 kg whereas for group B were 9.60, 20.13, -12.37, 22.52 cm, 10.36 mm, 12.10 kg and 22.82 kg. The difference was not statistically significant (Table 3). The distribution of study participants according to aetiology of cirrhosis were similar in two groups with alcohol as major cause 19 (63.33%) in group A and 24 (80.00%) in group B. The second most common aetiology was NASH 9(30%) in group A and 5 (16.67%) in group B. The other aetiology was autoimmune, Hepatitis B and Hepatitis C infection (Table 4). Table 5 shows effect of protein diet at the end of study period between two groups. The patients in group A had improved serum albumin level ˄ 0.29 / dl compared to group B with ˄ 0.05 which was statistically significant (p value- <.05).
* Unpaired ‘T’ Test, ** Mann-Whitney Rank Sum Test, *** Fisher Exact Test.
Chi-square = 4.724 with 4 degrees of freedom, P = 0.32.
The hand grip and skeletal muscle mass also showed improvement in group A 1.0 & 0.7 against group B with 0.07 & -0.02 respectively, the difference of both the parameters in the groups showed statistically significant difference (Table 5) {Hand grip p value .03, Skeletal muscle mass p value .048} CTP and MELD Na score also improved in group A (ΔCTP of -0.73 ± 1.46 and ΔMELD of -0.63 ± 2.17) whereas in group B, CTP improved by a mean of -0.23 ± 0.97 and MELD Na increased by 0.33 ± 2.93 but the difference between the two groups was not statistically significant (p = 0.25 for ΔCTP and p = 0.16 for ΔMELD). The nutritional parameters like MAC, TSF and handgrip improved in group A whereas MAC and TSF decreased, and hand grip improved marginally in group B but the difference between the groups was significant only for hand grip (ΔMAC of 0.06 cm ± 0.19 vs. -0.01 cm ± 0.18, p=0.17; ΔTSF of 0.01 mm ± 0.3 vs. -0.04 mm ± 0.18, p=0.41; ΔHG of 1.0 kgf ± 1.6 vs. 0.07 kgf ± 1.7, p = 0.03). Skeletal muscle mass improved significantly in group A while it decreased in group B (mean change of 0.07 kg ± 0.19 vs. -0.02 ± 0.15, p < 0.05).
* Unpaired ‘T’ Test, ** Mann-Whitney Rank Sum Test, ‘Δ’ – denotes change in value of variable (final – baseline).
When compared to baseline, patients in group A had significant decrease in fasting arterial ammonia level (-11.83μmol/l ± 24.57, p = 0.013) and significant improvement in PHES score (1.57 ± 2.36, p = 0.002) whereas group B patients did not show any significant changes in these parameters from baseline (Table 4), however when the improvement in group A was compared with group B the difference between the two groups was not statistically significant (p = 0.08 for ammonia and p = 0.11 for PHES) (Table 6). The HRQOL, assessed using SF-36 health survey questionnaire, improved to a greater extent in group A as compared to group B with statistically significant improvement noted in physical functioning (p = 0.03), role limitation due to physical health (p = 0.03), energy/fatigue (p = 0.04), general health (p = 0.04) and health change (p = 0.02) scale in group A patients. Other scales of HRQOL like role limitation due to emotional problems, emotional wellbeing, social functioning and pain scale, improved more in group A but the difference was not significant when compared to group B (Table 7).
* Unpaired ‘T’ Test, **Wilcoxon Signed Rank Test, *** Mann-Whitney Rank Sum Test, ‘Δ’ – denotes change in value of variable (final – baseline).
* Mann-Whitney Rank Sum Test, ‘Δ’ – denotes change in value of variable (final – baseline).
Discussion
This study showed that patients who were given optimal protein diet had significant improvement in serum albumin level, hand grip and skeletal muscle mass. Similar finding of significant increase in albumin level was also noted in the group of cirrhotic patients who were given dietary intervention [20-24]. And also noted a significant increase in hand grip and skeletal muscle mass but did not report any significant improvement in hand grip with BCAA supplementation in their study [25]. The improvement in skeletal muscle mass in intervention group had a significant negative correlation with fasting arterial ammonia level and a significant positive correlation with changes in MAC and hand grip. Similar finding was also noted with nutritional therapy in their patients with MHE. It also corresponds to the fact that skeletal muscle plays a role in ammonia detoxification, hence as muscle mass increases it could possibly lead to a higher clearance of ammonia.
Although other nutritional parameters like MAC and TSF increased in interventional group and decreased in control group, the difference between the two groups was not significant in present study. However, significant improvement was seen in MAC and in TSF of the respective studies. This discrepancy could be due to the fact that duration of study was significantly longer in these studies (14 months, 6 months, 12 months) as compared to this study (1 month) [26]. Indicators of severity of liver disease like CTP score and MELD Na decreased to a greater extent with dietary intervention while in control group CTP score decreased but MELD Na increased at the end of study period, though the difference in both parameters between the two groups was not statistically significant. And also, did not observe any significant improvement of CTP or MELD score in their study. They did find significant improvement in these parameters in their study, but the duration of their study was much longer (6 months and 12 months) and moreover, these studies had majority of patient in CTP class ‘B’ (70% and 63.2%) while in this study greater number of patients were in CTP class ‘C’ (51.7% in class ‘C’ and 45% in class ‘B’) [27].
HRQOL improved to a greater extent in patients given adequate protein supplementation with statistically significant improvement noted in most of the parameters. Similar improvement of HRQOL with dietary intervention was also noted. There were few limitations of the study like, small sample size, shorter duration of study and therefore further studies with a longer duration of followup are required to assess the effects of nutritional intervention on in cirrhotic liver disease. Another limitation was that Alcohol and NASH related cirrhosis together accounted for more than 90% of cases in the current study which might not give true representation of the whole aetiological spectrum of liver cirrhosis [28].
Conclusion
Liver cirrhosis causes an ill-balanced metabolic state which, in the setting of nutritional deprivation, predisposes to protein energy malnutrition ultimately increasing the morbidity and mortality in these patients. Nutritional assessment followed by nutritional therapy with optimal dietary vegetable protein is safe, effective and well tolerated, even in patients with hepatic encephalopathy and should be offered to cirrhotic patients to prevent the perils of widely prevalent malnutrition in these patients.
References
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, et al. (2014) Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 60(2): 715-735.
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, et al. (1999) Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 30(5): 890-895.
- Nabi E, Bajaj JS (2014) Useful Tests for Hepatic Encephalopathy in Clinical Practice. Curr Gastroenterol Rep 16(1): 362.
- Mendenhall C, Rinelle GA, Gartside P, Moritz T (1995) Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veteran Administration Cooperative studies. Alcohol Clin Exp Res 19(3): 635-641.
- Moller S, Bendtsen F, Christensen E, Henriksen JH (1994) Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J Hepatol 21(6): 940-946.
- Lautz HU, Selberg O, Korber J, Burger M, Muller MJ (1992) Protein-calorie malnutrition in liver cirrhosis. Clin Investig 70(6): 478-486.
- Pikul J, Sharpe MD, Lowndes R, Ghent CN (1994) Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation 57(3): 469-472.
- Alberino F, Gatta A, Amodio P, Merkel C, Pascoli LD, et al. (2001) Nutrition and survival in patients with liver cirrhosis. Nutrition 17(6): 445-450.
- Riordan SM, Williams R (1997) Treatment of hepatic encephalopathy. N Engl J Med 337: 473-479.
- Plauth M, Merli M, Kondrup J (1997) Management of hepatic encephalopathy. N Eng J Med 337: 1921-1922.
- Mullen KD, Weber Jr FL (1991) Role of nutrition in hepatic encephalopathy. Semin Liver Dis 11: 292-304.
- Cordoba J, Hellin JL, Planas M, Sabin P, Sanpedro F, et al. (2004) Normal protein diet for episodic hepatic encephalopathy: Results of a randomized study. J Hepatol 41(4): 38-43.
- Bass N (2007) Review article: The current pharmacological therapies for hepatic encephalopathy. Aliment Pharmacol Ther 25: 23-31.
- Huynh DK, Selvanderan SP, Harley HA, Holloway RH, Nguyen NQ (2015) Nutritional care in hospitalized patients with chronic liver disease. World J Gastroenterol 21(45): 12835-12842.
- Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, et al. (2019) ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin Nutr 38(2): 485-521.
- Ney M, Vandermeer B, van Zanten SJ, Ma MM, Gramlich L, et al. (2013) Meta-analysis: oral or enteral nutritional supplementation in Cirrhosis. Aliment Pharmacol Ther 37(7): 672-679.
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, et al. (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40(4): 423-429.
- Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, et al. (2010) Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci 55(8): 2381-2390.
- Ware JE, Sherbourne CD (1992) The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care 30(6): 473-483.
- Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, et al. (1990) Long-term oral branched-chain amino acids in advanced cirrhosis: a double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol 11(1): 92-101.
- Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, et al. (2007) BCAA enriched snack improves nutritional state of cirrhosis. Nutrition 23(2): 113-120.
- Cabre E, Huix FG, Lacruz AA, Esteve M, Acero D, et al. (1990) Effect of Total Enteral Nutrition on the Short-Term Outcome of Severely Malnourished Cirrhotics: A Randomized Controlled Trial. Gastroenterology 98(3): 715-720.
- Maharshi S, Sharma BC, Sachdeva S, Srivastava S, Sharma P (2016) Efficacy of Nutritional Therapy for Patients with Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol 14(3): 454-460.
- Bories PN, Campill B (1994) One-month regular oral nutrition in alcoholic cirrhotic patients; Changes of nutritional status, hepatic function and serum lipid Pattern. Br J Nutr 72(6): 937-946.
- Les I, Doval E, Garcia-Martinez R, Planas M, Cardenas G, et al. (2011) Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol 106(6): 1081-1088.
- Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, et al. (2003) Nutritional supplementation with branched chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 124(7): 1792-1801.
- Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, et al. (2005) Effects of oral branched chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 3(7): 705-713.
- Kato A, Tanaka H, Kawaguchi T, Kanazawa H, Iwasa M, et al. (2013) Nutritional management contributes to improvement in minimal hepatic encephalopathy and quality of life in patients with liver cirrhosis: A preliminary, prospective, open-label study. Hepatol Res 43(5): 452-458.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...