Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Review Article(ISSN: 2641-1652) 
Oral Agents in the Treatment of Inflammatory Bowel Disease: A Remarkable Progress Volume 3 - Issue 4
Andrew Herman1,2 and Atilla Ertan1,2*
- 1The Ertan Digestive Disease Center, USA
- 2Gastroenterology, Hepatology and Nutrition Division University of Texas Health Science Center at Houston McGovern Medical School, USA
Received:March 10, 2022 Published: March 14, 2022
*Corresponding author: Atilla Ertan, The Ertan Digestive Disease Center, Gastroenterology, Hepatology and Nutrition Division University of Texas Health Science Center at Houston McGovern Medical School, Texas, USA.
DOI: 10.32474/CTGH.2022.03.000167
Abstract
Despite remarkable progress for management of moderate-to-severe IBD patients, there are significant rates of primary nonresponse, loss of response, and/or adverse events thereby necessitating additional treatment options. Additionally, the burden of intravenous administration or subcutaneous injection of biologics accompanied by associated high cost necessitate the development of alternative treatments. In recent years, remarkable research has focused on the development of oral small molecule agents. Fortunately, the rapidly growing number of targeted therapies with oral small molecule agents offer the advantage of ease of administration with the durable effectiveness and no downside of immunogenicity with relatively more safety profile compared to the FDA approved biologic agents with relatively lower cost in the management of patients with moderate-to-severe IBD. The purpose of this review article is to summarize available novel oral small molecule agents in treatment of patients with IBD. Two of the oral small molecule agents, tofacitinib and ozanimod were already approved by the FDA for patients with moderate-to-severe ulcerative colitis. We will not include other emerging therapeutic modalities such as microbiome targeted or stem cell therapies in this review.
Introduction
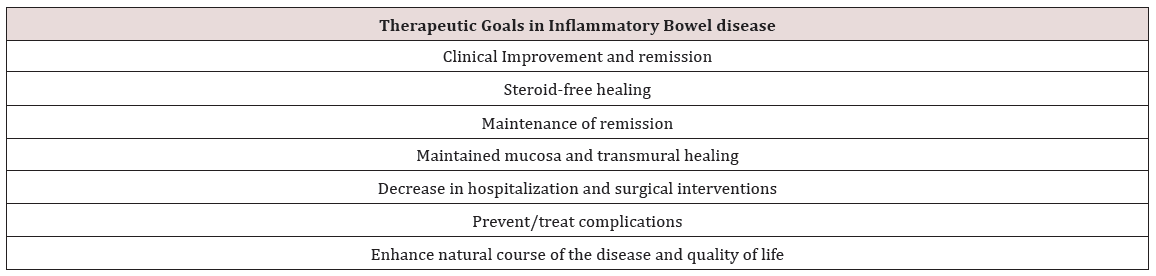
Inflammatory bowel disease (IBD) including ulcerative colitis and Crohn’s disease are chronic relapsing disorders of intestinal inflammation which leads to decreased quality of life, disability, and bowel damage resulting in hospitalizations and surgeries [1]. Several genetic models have been developed for the inflammatory cascade that leads to the chronic inflammation seen in IBD, but none of these models have been able to account for all the observed pathophysiologic features. This complexity is probably secondary to the intricate nature of IBD and an incomplete understanding of the interactions between the mucosal immune system and the intestinal microbiota [2]. The current goal of treatment is to achieve clinical, endoscopic and histologic remission of disease activity. Management of IBD is generally divided into induction and maintenance phases. These phases involve achieving remission of inflammation quickly usually over a 2-3 month period and then maintenance of that clinical and histologic remission beyond that point (Table 1).
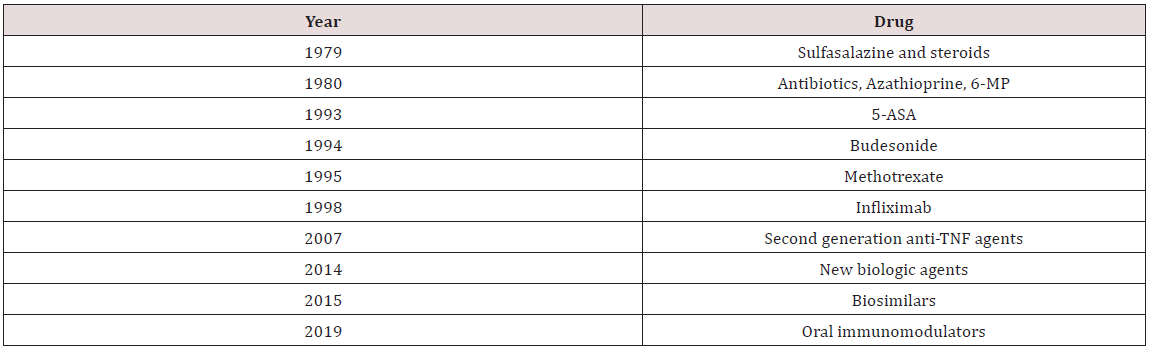
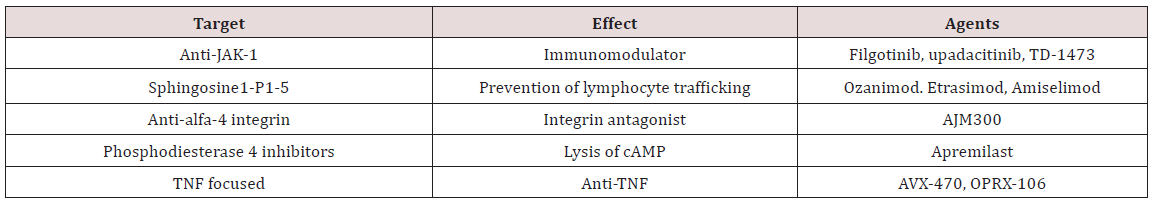
5-ASA acts as a topical anti-inflammatory agent that has efficacy within the lumen of the intestine [3]. Although its use is well established in ulcerative colitis, its efficacy in its effect in Crohn’s disease is not better than placebo. Mild-to-moderate ulcerative colitis can be managed with oral 5-ASA treatments, but is not effective for moderate-to-severe disease. Older treatments for moderate-to-severe IBD include steroids and thiopurines including azathioprine, mercaptopurine, and methotrexate (Table 2). Despite the efficacy of prednisone in improving acute symptoms of IBD patients, they have not consistently demonstrated effectiveness in controlling histologic inflammation. In addition, side effects related to long term use of steroids can be debilitating including but not limited to insomnia, personality changes, acne, fatigue, and weight gain. Alternative agents such as the oral controlled ileal release budesonide have been developed. Budesonide is efficacious in the management of inflammation in the terminal ileum and right colon. This makes budesonide effective for short-term relief of symptoms for mild-to-moderate IBD but not a good long-term option. The efficacy of thiopurines to maintain medically induced remission as well as to prevent post-operative recurrence in IBD has been well established. In the SONIC trial, the mucosal healing rate in patients in clinical remission was not significantly different between the azathioprine and anti-TNF monotherapy arms (36% vs. 43%) [4]. Despite their effectiveness many patients elect to stop thiopurine therapy. Up to 40 percent of patients discontinued thiopurine therapy in the first 4 months of treatment due to intolerance or ineffectiveness [5]. In addition, there is no doubt that thiopurines safety profile is inferior as there is a significant risk of developing lymphoma and non-melanoma skin cancer [6]. No satisfactory management of IBD was achieved prior to the development of infliximab. Current recommendations for the management of IBD with biologics include the use of early therapy with a treat-to target strategy to achieve clinical remission, mucosal and histologic healing which ultimately decrease the risk of corticosteroid use, surgeries, hospitalizations and increases quality of life [7]. Available biologic agents include anti-tumor necrosis factor alpha (TNF-a) agents including infliximab, adalimumab, certolizumab, and golimumab; anti-integrin agents including vedolizumab and natalizumab; and anti-interleukin (IL) 12-23 agents such as ustekinumab. Unfortunately, despite remarkable progress in the management of moderate-to-severe IBD patients, there are significant rates of primary non-response, loss of response, and adverse events, thereby necessitating additional treatment options. Additionally, the burden of intravenous administration or subcutaneous injection accompanied by associated high cost necessitate the development of alternative treatments. In recent years, remarkable research has focused on the development of oral small molecule agents (Table 3). Unlike antibodies that can develop with biologic agents, oral small molecule formulations do not carry the same risk of immunogenicity. Their molecular characteristics and size allow for a more convenient oral administration and avoids the potential development of anti-drug antibodies. The purpose of this review article is to summarize available novel oral small molecule agents in treatment of patients with IBD. Two of the oral small molecule agents, tofacitinib and ozanimod were already approved by the FDA for patients with moderate-to-severe ulcerative colitis. This review will not include other novel and emerging therapeutic modalities such as microbiome targeted or stem cell therapies.
JAK Inhibitors
One of the new treatment strategies that has been developed is the targeting of the Janus kinase (JAK) family of tyrosine kinases [8]. The functions of this family of tyrosine kinases is broad, but evidence suggests that innate and adaptive immune responses require JAK-STAT signaling to mediate several pathways of cytokine function. These agents inhibit cytokines in the inflammatory cascade such as IL-9, IL-12, IL-23 and interferon-gamma. Several studies have supported this hypothesis by demonstrating significant upregulation of JAK transcripts in intestinal mucosa of patients with active ulcerative colitis [9]. These facts make targeting the JAK-STAT an appealing therapeutic modality in IBD. This has led to the development and regulatory approval by the United States Food and Drug Administration (FDA) of therapies targeting the JAK pathway for the treatment of IBD [10]. One of those therapies is a non-specific pan-JAK inhibitor tofacitinib, which was approved by the FDA in 2018 for the treatment of patients with moderateto- severe ulcerative colitis. The Octave trial which included the phase 3 clinical trial that led to the regulatory approval of tofacitinib for the treatment of ulcerative colitis included patients randomly assigned to 10 mg of tofacitinib twice daily or placebo for 8 weeks. Clinical remission (determined to be a Mayo Clinic score of less than 2 and a rectal bleeding score of 0) at 8 weeks occurred in 18.5% of patients assigned to tofacitinib versus 8.2% of patients assigned to placebo (P = 0.007) [11]. The Octave sustain maintenance study re-randomized week 8 responders to receive 10 mg or 5 mg of maintenance tofacitinib twice daily or placebo for 52 weeks. Remission at 52 weeks was significantly higher in patients treated with 5 mg (34.3%) and 10 mg (40.6%) of tofacitinib than with placebo (11.1%; p<0.001 for both comparisons with placebo). Notably, tofacitinib has a rapid onset of induction and has shown to be effective in refractory anti-TNF exposed ulcerative colitis patients compared to placebo [12]. Similar studies in patients with Crohn’s disease failed to achieve primary and secondary endpoints though there were modest improvements in inflammatory markers; this was probably due to the study design and unusually high placebo response rate seen in the study [13]. Clearly more research in the use of tofacitinib is needed to elucidate its efficacy in patients with Crohn’s disease. This data makes tofacitinib an attractive treatment option for patients with moderate-to-severe ulcerative colitis. However, tofacitinib can inhibit the immune system to a degree that increases the risk of herpes zoster, serious bacterial infections, tuberculosis, and upper respiratory tract infections [14]. The safety committee of the European Medicines Agency performed a review of tofacitinib due to the concern for an increased risk of developing pulmonary embolisms [15]. Subsequently, the FDA released warnings about the risk of blood clots leading to a boxed warning. All patients irrespective of their risk factors for developing thromboembolism should be monitored for signs and symptoms of pulmonary emboli. Sudden death in patients using high doses of tofacitinib was seen in patients primarily with rheumatoid arthritis and not with inflammatory bowel disease [16,17]. Another important concern related to the use of tofacitinib is the increased risk of lymphoma and nonmelanoma skin malignancies [18]. In a recent study, 1455 patients receiving tofacitinib at a dose of 5 mg twice daily and 1456 patients receiving tofacitinib at a dose of 10 mg twice daily were compared to 1451 patients receiving a TNF inhibitor. Over a 4 year follow up period, incidences of cancer and major cardiovascular events were higher in patients receiving the combined tofacitinib doses (4.2% and 3.4%, respectively) than with a TNF-alpha inhibitor (2.9% and 2.5%) [19]. The hazard ratios were 1.33 for major cardiovascular events and 1.48 for cancer (particularly non-melanoma skin cancer); the non-inferiority of tofacitinib was not demonstrated. Rarely, gastrointestinal perforations can occur during tofacitinib therapy with data demonstrating and incidence of 0.2% [20]. It is important to remember that patients should be advised to reduce their dose of tofacitinib in half when combining treatment with cytochrome P450 inhibitors such as fluconazole and ketoconazole.
Selective JAK inhibitors have also been recently studied for the treatment of patients with IBD, including Crohn’s disease. Filgotinib, is a selective JAK inhibitor that was approved by the FDA for the treatment of rheumatoid arthritis. Filgotinib selectively targets the JAK1 cytokine at a 30-fold selectivity over JAK2. JAK2 inhibition is thought to lead to higher rates of anemia and thrombocytopenia through the interfering of erythropoietin and thrombopoietin and granulocyte-macrophage colony-stimulating factor which would make selective JAK1 inhibition an attractive option [21,22]. The phase II Fitzroy study demonstrated early clinical benefit of filgotinib in patients with Crohn’s disease. Fitzroy included patients with a disease activity score (CDAI) of 220-450 and confirmed endoscopically active Crohn’s disease. A total of 174 patient with moderate-to-severe active Crohn’s disease were randomly assigned to receive 200 mg of filgotinib daily or placebo for 10 weeks. Clinical remission (indicated as a CDAI score of <150) at 10 weeks was achieved in 47% of patients treated with filgotinib and 23% of patients who were given placebo (P = 0.0077). Endoscopic improvement at 10 weeks was not significantly different [23]. The phase II study, Divergence 2, examined the effect of filgotinib in patients with perianal fistulizing Crohn’s disease. Patients with a documented history or perianal fistulizing Crohn’s disease with at least one to two external openings with drainage previously treated with immunomodulators or anti-TNFs were randomly assigned to receive filgotinib 200 mg, 100 mg or placebo once daily for 24 weeks. The primary endpoint was fistula response with a reduction of greater than 1 from baseline in the number of fistulas and no fluid collections seen on MRI at week 24. Unfortunately, the study was not well powered as there was low recruitment rates due to the COVID-19 pandemic leading to a total of 57 participants. Results did demonstrate a numerically higher proportion of patients in the filgotinib 200 mg group (47%) versus the placebo group (25%) who achieved the primary endpoint [24]. Further studies with a large patient population will be required to further elucidate the efficacy of filgotinib in patients with fistulizing Crohn’s disease.
The Selection trial included two induction studies, a maintenance study, and a long- term extension study examining the efficacy of filgotinib in the treatment of moderate-to-severe ulcerative colitis. Adults with moderate-to-severe ulcerative colitis were randomized to filgotinib 200 mg, 100 mg or placebo once daily for 11 weeks. Patients who responded to selected treatment at week 10 were re-randomized to continue filgotinib or placebo for an additional 47 weeks. Clinical remission was evaluated at week 10 and 58. Filgotinib demonstrated clinical remission rates significantly improved over placebo (47% vs 23%) at 10 weeks. Secondary endpoints such as endoscopic remission, mucosal healing, and deep remission did achieve numerical improvement but failed to achieve statistical significance. At week 58 remission was achieved at a rate of 58% in the filgotinib group compared to placebo at 29.5%. These findings suggest that filgotinib is efficacious at inducing and maintaining remission in patients with ulcerative colitis. Due to the efficacy demonstrated by filgotinib regulatory approval for use in moderate-to-severe ulcerative colitis as well as Crohn’s disease is expected soon. The common side effects reported in patients taking filgotinib are quite similar to tofacitinib including but not limited to serious infections, herpes zoster, venous thrombosis, pulmonary embolism and gastrointestinal perforations [25,26]. It is of note that filgotinib was rejected for approval by the FDA in the treatment of rheumatoid arthritis on concerns of toxicity and reduced sperm count [27,28]. Two ongoing trials (MANTA and MANTA-Ray) are pending and will provide additional safety data on the matter in patients with IBD.
Another highly selective JAK inhibitor that was approved by the FDA for use in patients with rheumatoid arthritis, psoriatic arthritis, and atopic dermatitis and also has been studied for its potential benefit in IBD patients is upadacitinib. This molecule is even more selective for JAK1 then filgotinib and has been investigated in the Celest phase 2 trial [29,30]. Patients with ulcerative colitis who had been previously exposed to anti-TNFs were evaluated after 16 weeks for primary endpoints of clinical remission, which included a patient reported outcome of stool frequency and abdominal pain score. In the induction phase of the study, although a numerical benefit in clinical remission could be observed in the group on twice daily 6 mg upadacitinib, it did not demonstrate a statistical response over placebo. The phase II study showed that the clinical remission and endoscopic improvement were achieved better than placebo as well as reduction in inflammatory markers. Phase III clinical trials of upadacitinib are ongoing and will hopefully shed more light on its efficacy and risk profile. Upadacitinib has similar adverse events as seen with tofacitinib including major adverse cardiovascular events and serious infections [31]. Due to the concerns of significant adverse events with tofacitinib, filgotinib, and upadacitinib, the development of a more gut selective pan-JAK inhibitor has been investigated. TD-1473 is a pan-JAK inhibitor that has demonstrated such a gut selective effect on mice with in-vitro studies [32]. A Phase 2b/3 set of clinical trials is currently ongoing to assess the efficacy and safety of induction and maintenance therapy with TD-1473 in subjects with moderate-to-severe active ulcerative colitis. Preliminary results were promising in endoscopic improvement along with reduction in fecal calprotectin and CRP levels with TD-1473 compared to placebo. If its further effectiveness can be demonstrated with this pending trial it has the potential to limit severe systemic side-effects caused by other non-GI selective JAK inhibitors. TYK2 is one of the JAK-STAT family proteins that is involved in intracellular cytokine signaling and inhibition of which blocks IL-12, IL-23 and IFN [33]. The oral TYK2/JAK1 inhibitor is well tolerated and more selective than other JAK inhibitors potentially limiting toxicity. Two oral TYK2/JAK1 inhibitors, deucravacitinib and brepocitinib, are currently recruiting in phase II clinical trials for the treatment of moderate-to-severe ulcerative colitis and also, Crohn’s disease.
Sphingosine-1-Phosphate Receptor Modulators
Sphingosine 1- phosphate (S1P) receptors are G protein coupled receptors (S1P1-S1P5) that regulate the response and function of various cellular and organ systems including cell migration, proliferation, immune response, and trafficking of T and B lymphocytes from lymphoid organs [34,35]. Their role in the ability of immune cells to migrate to inflamed tissues has made them a potential new target of inhibition for the management of IBD. Ozanimod is a new oral small molecule agent that binds with high affinity to several S1P receptor subtypes leading to internalization of the receptor in targeted lymphocytes and prevention of lymphocyte trafficking [36]. Ozanimod was approved for the treatment of the patients with relapsing multiple sclerosis in 2020 and then for patients with moderate-to-severe ulcerative colitis in 2021 by the FDA. Sandborn et al performed a phase III double blind and placebo-controlled trial of ozanimod as induction and maintenance therapy in patients with moderate-to-severe ulcerative colitis [37]. Patients were assigned to receive oral ozanimod 1 mg or placebo once daily and patients in a second cohort received open label ozanimod. They found that clinical remission was significantly higher in patients who received ozanimod than those who were on placebo (37% vs 18.5%, P<0.001). Clinical response was also significantly higher in the ozanimod group (60% vs 41%, P<0.001). The investigators found that rates of serious infection were equal in both groups. A few patients on ozanimod had higher rates of elevated liver transaminases. Adverse events have been reported with ozanimod treatment including herpes zoster, bradycardia, and elevation of liver enzymes, atrioventricular conduction delays and macula edema. Ozanimod is also being studied for the treatment of Crohn’s disease. In the phase II Stepstone study involving 69 patients with Crohn’s disease, 39.1% of patients who received ozinamod had clinical remission at week 12. There was no incidence of bradycardia or arrhythmias in these patients [38]. Phase III, placebo-controlled induction and maintenance studies of ozanimod are currently recruiting for moderate-to-severe Crohn’s disease. Etrasimod is another oral S1P receptor subtype 1 modulator which has demonstrated potential efficacy in the treatment of patients with IBD. In the phase II Elevate trial involving 156 patients with ulcerative colitis, those patients receiving a 2 mg dose of etrasimod demonstrated endoscopic improvement over placebo (41.8% vs 17.8%, P=0.003). Also, compared to placebo, the etrasimod 2 mg group had a higher rates histologic remission (19.5% vs 6.1%; p=0.03). Etrasimod adverse events were reported as minimal with a small group of patients developing a transient, asymptomatic, low grade atrioventricular block that resolved spontaneously. Amiselimod is an oral S1P receptor modulator with higher selectivity for S1PR1 than other S1P receptor modulators [39]. A phase II trial with this agent is pending in patients with active Crohn’s disease.
Anti-Adhesion Molecules
Migration of proinflammatory T cells into the gut facilitates inflammation that is characteristic of IBD [40]. Anti-adhesion agents that block lymphocyte trafficking to the gut are being investigated in patients with IBD. A variety of oral small molecules including alfa-4 integrin antagonists have been studied. AJM300 is an oral small molecule agent that targets alfa-4 integrin. A phase II study in 102 patients with moderate-to-severe ulcerative colitis showed higher rates of clinical response (62.7% vs 25.5%, p=0.0002), clinical remission (23.5% vs 3.9%, p= 0.0099) and mucosal healing (58.8% vs 29.4%) [41]. No major adverse events were reported. A phase III trial is ongoing with AJM300 in patients with ulcerative colitis. There is extensive research of other anti-adhesion agents in the treatment of inflammatory bowel disease.
Phosphodiesterase 4 Inhibitors
Phosphodiesterase 4 (PDE4) is part of a group of enzymes that catalyze the breakdown of cyclic adenosine monophosphate (cAMP). In inflammatory cells, PDE4 is the dominant enzyme responsible for this reaction and the resulting decrease in cAMP levels leads to an increase expression of proinflammatory factors. Thus, it has been postulated that if PDE4 were inhibited the resulting increase in cAMP levels would lead to the decreased expression of a number of proinflammatory factors including TNF-alfa, IL- 17, IL-23, and up-regulates anti-inflammatory IL-10 [42]. This makes PDE4 a potential target for the treatment of inflammatory disorders. Apremilast is an oral small molecule PDE4 inhibitor which has been approved by the FDA for the treatment of adults with psoriatic arthritis, plaque psoriasis, and Behcet’s disease. A recent phase II clinical trial demonstrated the clinical effectiveness of apremilast in the treatment of moderate-to-severe ulcerative colitis. The investigators performed a double-blind, placebocontrolled trial in patients with active ulcerative colitis who were either biologic naïve or had failed conventional therapies. Patients were randomly assigned to apremilast 30 mg twice daily, 40 mg twice daily, or placebo for 12 weeks. After which patients were then randomly assigned to receive apremilast 30 mg or 40 mg twice daily for an additional 40 weeks. Endoscopies were performed and biopsies were obtained at the initial encounter, week 12, and week 52 after initiation of the study. The primary endpoint for the study was clinical remission at week 12 (a Mayo score of 2 or less). The investigators found that clinical remission was achieved in the 30 mg apremilast group at a rate of 31.6% versus 12.1% of patients in the placebo group (P = 0.01) [43]. Both apremilast groups (the 30 mg and 40 mg groups) had similar improvement from baseline in Mayo score components. At week 52 clinical remission was achieved by 40.4% of patients. Endoscopic healing was achieved in 41.4% of patients in placebo compared to 73.7% in the 30 mg group (p< 0.0001). Moreover, both the 30 mg and 40 mg apremilast groups showed greater reduction in serum C-reactive protein and fecal calprotectin compared to placebo. In terms of safety, headache and nausea were found to be the most common side effect. One patient had an episode of acute pancreatitis but this was no attributed to the study drug. Currently, a phase III trial has not been registered for patients of ulcerative colitis or Crohn’s disease.
Anti-Tumor Necrosis Factor Agents
Anti-TNF agents were the first class of biologic medications approved for the treatment of patients with inflammatory bowel disease [44]. Limitations of this class of medications includes the intravenous or subcutaneous administration, infusion reactions, systemic side effects related to immunosuppression, and high cost [45]. An oral agent with a mechanism of action restricted to the gastrointestinal tract would be helpful to overcome some of these challenges of parenterally administered anti-TNF agents. AVX- 470 is an oral polyclonal immunoglobulin that inhibits TNF-alpha locally in the gastrointestinal tract, minimizing systemic exposure [46]. In a double blind, placebo-controlled trial, 37 patients with active ulcerative colitis received AVX-470 (0.2, 1.6, or 3.5 grams per day) or placebo for 4 weeks. Endoscopic activity was assessed pre and post treatment exposure.46 At all AVX-470 doses, 25.9% of patients achieved clinical response compared with 11.1% of those in the placebo group. Both groups were found to have similar adverse event rates without significant infectious reported.46 Further clinical trials evaluating the efficacy of AVX-470 are ongoing [47,48]. OPRX-106 is another oral anti-TNF agent which is currently undergoing evaluation for its efficacy in the treatment of inflammatory bowel disease [49]. In a phase II randomized open label clinical trial, 25 patients with ulcerative colitis who were administered OPRX-106 demonstrated clinical remission and mucosal healing with no major adverse events including immunogenicity. Initial studies with oral anti-TNF agents have shown promising results with the potential for enhanced safety and decreased immunogenicity. However, larger trials are needed to evaluate efficacy, safety and cost effectiveness.
Conclusion
Despite tremendous advancements in the field of treatments for IBD, there are significant rates of primary non-response, loss of response, adverse events and high cost thereby necessitating additional treatment options. Fortunately, the rapidly growing number of oral small molecule targeted therapies offers ease of administration with durable effectiveness, and an improved safety profile compared to the currently approved therapeutic agents.
Acknowledgement
Our investigators at the Ertan Digestive Disease Center were and still are principal investigators during pre-FDA approval of research trials with tofacitinib, ozanimod, filgotinib and etrasimod. This study is supported by the Ertan Research and Education Foundation.
Disclosures
Dr Ertan has research grant supports by the AbbVie, Celgene Corp, Gilead Sciences, Pfizer, Eli Lilly and Takeda pharmaceuticals.
Conflict of Interest
The authors declare that the manuscript was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Al-Bawardy B, Shivashankar R, Proctor DD (2021) Novel and Emerging Therapies for Inflammatory Bowel Disease. Front Pharmacol 12: 651415.
- Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, et al. (2018) ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol 113(4): 481-517.
- Baumgart DC, Carding SR (2007) Inflammatory bowel disease: cause and immunobiology. Lancet 369(9573): 1627-1640.
- Peyrin-Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, et al. (2014) Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 63(1): 88-95.
- Targownik LE, Leung S, Lix LM, Singh H, Bernstein CN (2018) Persistence With Immunomodulator Monotherapy Use And Incidence of Therapeutic Ineffectiveness Among Users of Immunomodulator Monotherapy in IBD. Am J Gastroenterol 113(8): 1206-1216.
- Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, et al. (2012) Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 143(2): 390-399 e1.
- Klenske E, Bojarski C, Waldner M, Rath T, Neurath MF, et al. (2019) Targeting mucosal healing in Crohn's disease: what the clinician needs to know. Therap Adv Gastroenterol 12: 1756284819856865.
- Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, et al. (2020) JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17(6): 323-337.
- Cordes F, Foell D, Ding JN, Varga G, Bettenworth D (2020) Differential regulation of JAK/STAT-signaling in patients with ulcerative colitis and Crohn's disease. World J Gastroenterol 26(28): 4055-4075.
- D'Amico F, Parigi TL, Fiorino G, Biroulet LP, Danese S (2019) Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol 12: 1756284819848631.
- Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, et al. (2017) Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 376: 1723-1736.
- Lair-Mehiri L, Stefanescu C, Vaysse T, Laharie D, Roblin X, et al. (2020) Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis 52(3): 268-273.
- Panes J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, et al. (2017) Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut 66(6): 1049-1059.
- Honap S, Chee D, Chapman TP, Patel M, Kent AJ, et al. (2020) Real-world Effectiveness of Tofacitinib for moderate-to-severe Ulcerative Colitis: A Multicentre UK Experience. Journal of Crohn's and Colitis 14(10): 1385-1393.
- Sabine Straus (2019) Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC). Pharmacovigilance Risk Assessment Committee (PRAC) p. 71.
- Xeljanz, Xeljanz XR (2019) FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). In: Administration FaD.
- Xeljanz, Xeljanz XR (2019) Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. In: Services TUSDoHaH, ed.
- Liscinsky M (2012) FDA approves Xeljanz for rheumatoid arthritis. In: Administration TUSFaD, ed.
- Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, et al. (2022) Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med 386: 316-326.
- Sandborn WJ, Panes J, D'Haens GR, Sands BE, Su C, et al. (2019) Safety of Tofacitinib for Treatment of Ulcerative Colitis, Based on 4.4 Years of Data From Global Clinical Trials. Clin Gastroenterol Hepatol 17(8): 1541-1550.
- Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG, et al. (2019) Upadacitinib and filgotinib: the role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context 8: 212595.
- Van Rompaey L, Galien R, van der Aar EM, Lacroix PC, Nelles L, et al. (2013) Preclinical Characterization of GLPG0634, a Selective Inhibitor of JAK1, for the Treatment of Inflammatory Diseases. The Journal of Immunology 191(7): 3568-3577.
- Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, et al. (2017) Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389(10066): 266-275.
- Reinisch W, Colombel JF, D’Haens GR, Rimola J, Amatsaleh AD, et al. (2022) OP18 Efficacy and safety of filgotinib for the treatment of perianal fistulizing Crohn’s Disease: Results from the phase 2 DIVERGENCE 2 study. Journal of Crohn's and Colitis 16: i019-i021.
- Schreiber S, Loftus EV, Jr, Maaser C, Danese S, Rudolph C, et al. (2022) DOP37 Efficacy and safety of filgotinib in patients with Ulcerative Colitis stratified by age: Post hoc analysis of the phase 2b/3 SELECTION and SELECTIONLTE studies. Journal of Crohn's and Colitis 16: i085-i087.
- Feagan BG, Danese S, Loftus EV, Jr, Vermeire S, Schreiber S, et al. (2021) Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 397:2372-2384.
- Mullard A. (2021) 2020 FDA drug approvals. Nat Rev Drug Discov 20(2): 85-90.
- Dhillon S, Keam SJ (2020) Filgotinib: First Approval. Drugs 80(18): 1987-1997.
- Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, et al. (2020) Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 158(3): 550-561.
- Parigi TL, D'Amico F, Danese S (2021) Upadacitinib for Crohn's Disease and Ulcerative Colitis Treatment: Hitting the Selective JAKpot. Gastroenterology 160(5): 1472-1474.
- Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, et al. (2018) Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391(10139): 2503-2512.
- Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, et al. (2020) Development of Gut-Selective Pan-Janus Kinase Inhibitor TD-1473 for Ulcerative Colitis: A Translational Medicine Programme. J Crohns Colitis 14(9): 1202-1213.
- Danese S, Peyrin-Biroulet L (2021) Selective Tyrosine Kinase 2 Inhibition for Treatment of Inflammatory Bowel Disease: New Hope on the Rise. Inflamm Bowel Dis 27(12): 2023-2030.
- Moolenaar WH, Hla T (2012) SnapShot: Bioactive lysophospholipids. Cell 148: 378-378.e2.
- Gardell SE, Dubin AE, Chun J (2006) Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med 12(2): 65-75.
- Senussi NH, Rakov N (2022) Ozanimod for Ulcerative Colitis. N Engl J Med 386: 194-195.
- Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, et al. (2021) Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 385: 1280-1291.
- Feagan BG, Sandborn WJ, Danese S, Wolf DC, Liu WJ, et al. (2020) Ozanimod induction therapy for patients with moderate-to-severe Crohn's disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol 5(9): 819-828.
- Sugahara K, Maeda Y, Shimano K, Mogami A, Kataoka H, et al. (2017) Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br J Pharmacol 174(1): 15-27.
- Adams DH, Eksteen B (2006) Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 6(3): 244-251.
- Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, et al. (2015) Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 149(7): 1775-1783.e2.
- Page CP, Spina D (2011) Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol 204: 391-414.
- Danese S, Neurath MF, Kopon A, Zakko SF, Simmons TC, et al. (2020) Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients With Active Ulcerative Colitis. Clin Gastroenterol Hepatol 18(11): 2526-2534 e9.
- Kornbluth A (1998) Infliximab approved for use in Crohn's disease: a report on the FDA GI Advisory Committee conference. Inflamm Bowel Dis 4(4): 328-329.
- Desai SB, Furst DE (2006) Problems encountered during anti-tumour necrosis factor therapy. Best Pract Res Clin Rheumatol 20(4): 757-790.
- Harris MS, Hartman D, Lemos BR, Erlich EC, Spence S, et al. (2016) AVX-470, an Orally Delivered Anti-Tumour Necrosis Factor Antibody for Treatment of Active Ulcerative Colitis: Results of a First-in-Human Trial. J Crohns Colitis 10(6): 631-640.
- Hartman DS, Tracey DE, Lemos BR, Erlich EC, Burton RE, et al. (2016) Effects of AVX-470, an Oral, Locally Acting Anti-Tumour Necrosis Factor Antibody, on Tissue Biomarkers in Patients with Active Ulcerative Colitis. J Crohns Colitis 10(6): 641-649.
- Weisshof R, El Jurdi K, Zmeter N, Rubin DT (2018) Emerging Therapies for Inflammatory Bowel Disease. Adv Ther 35(11): 1746-1762.
- Almon E, Shaaltiel Y, Sbeit W, Fich A, Schwartz D, et al. (2021) Novel Orally Administered Recombinant Anti-TNF Alpha Fusion Protein for the Treatment of Ulcerative Colitis: Results From a Phase 2a Clinical Trial. J Clin Gastroenterol 55(2): 134-140.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...