Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Review Article(ISSN: 2641-1652) 
Non-Alcoholic Fatty Liver Disease and its Interplay with Various Metabolic Disorders Volume 3 - Issue 1
Ishimwe Steven Papy1, Xiang-Rong Cheng1,2,3*, Guo-Wei Le1,2,3 and Md. Serajul Islam2
- 1School of Food Science and Technology, Jiangnan University, P.R. China
- 2National Engineering Research Center for Functional Food, Jiangnan University, PR China
- 3Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, Jiangnan University, PR China
Received: February 20, 2020; Published: March 03, 2020
*Corresponding author: Xiang-Rong Cheng, School of Food Science and Technology, National Engineering Research Center for Functional Food, Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, Jiangnan University, Wuxi, Jiangsu 214122, PR China
DOI: 10.32474/CTGH.2020.03.000152
Abstract
Nonalcoholic fatty liver disease (NAFLD) is described as exposition of multiplex liver metabolic disturbance interconnected with obesity. NAFLD is depicted by steatosis, excessive accumulation of fats in liver, due to triglycerides export and oxidation of fatty acid from plasma and de novo synthesis. Hepatic steatosis can therefore be explained as biochemical outcome of inconsistency between interfused mechanisms of lipid biotransformation. This condition is allied to a range of various modifications in lipoproteins, fatty acids, and glucose metabolisms in organism. So, above metabolic disfunctions are suspected to be the origin of possibility for adverse cardiometabolic risk agents related to NAFLD, like dyslipidemia, Type 2 diabetes mellitus (T2DM), and insulin resistance. Reactive oxygen species (ROS) generation participates as known inducer of inflammation and oxidative stress, that exacerbate this disease. These disorders are hallmarks that worsen NAFLD complications, so far participate in developing advanced stages of NAFLD and incline the body to CVD and T2D. The reciprocal risks exist among these diseases. Given the sharp growing prevalence and persistence of NAFLD, and its complexity that provoke additional metabolic syndrome, this review discusses various mechanisms of developing NAFLD, interaction with other associated hallmarks, aiming to clarify beneficial mechanisms for improvement.
Keywords: Nonalcoholic fatty liver disease; oxidative stress; Type 2 diabetes; noncoding RNAs; cardiovascular disease
Introduction
The liver is known as metabolically complex organ due to its
multiple biological activities, involving detoxification of various
endogenous metabolites and exogenous toxic substances, formation
of biochemicals needed for degradation and the synthesis of
protein. The liver also serves an exigent task in metabolisms like
lipid homeostasis and glycogen storage regulation [1]. Increased
activity of mitochondria consequent to the fatty acids hyper-afflux
from oxidation of fatty acid, produce free radicals that generate
liver oxidative stress [2]. Abnormal function of liver may engender
several metabolic impairments, such as NAFLD (Marrero et al.,
2002). NAFLD is explained as the grouping of excess fat into liver
cells, that does not result from alcohol consumption. The existence
of fats in the liver is normal; however, if the concentration of fats
exceeds 5%-10% of the weight of liver, then it is called a fatty liver
(steatosis). Thus, NAFLD is explained as the amassing of liver fat
of >5% of liver weight with <10g of daily alcohol consumption
(Bayne, 2010). Besides these, generation of NAFLD was discovered
to be linked with insulin resistance, the latter is known also as a
critical risk factor for developing type 2 diabetes (T2D) [3]. NAFLD
comprise a large spectrum of manifestations extending from simple
steatosis, continuing to nonalcoholic steatohepatitis (NASH) and
cirrhosis. Furthest, NAFLD correlates with significant higher risk
of developing hepatocellular carcinoma (HCC) [4]. The worse
hallmark of this disease is the significant interdependence with
various attributes of metabolic syndrome (MetS), such (T2DM),
obesity or dyslipidemia [5]. MetS is hallmarked by the aggregation
of several impairments involving elevated blood pressure, obesity,
dyslipidemia, insulin resistance and proinflammatory activities
[6]. Moreover, studies by different researchers elucidated NAFLD
as the leading inducer of liver diseases and the main reason for
impaired liver function worldwide; also considered as an inherent part of MetS (hypertension, hyperglycemia, central obesity and
dyslipidemia) [7,8]. The evolution of MetS has been coincided with
a growth in liver disorders including NAFLD, and was revealed
to correspond with disorders, like cardiovascular disease (CVD).
Particularly, NAFLD has been taken as hepatic exposition of MetS
[9].
The NAFLD prevalence increases quickly around the world
and noted as a camouflaged epidemic. The Universal estimation
of NAFLD prevalence in general population has overpassed 25%
[10], See Figure 1. In developing countries such as, China; and
India, the number of NAFLD patients has been augmenting sharply
over the last decades [11]. However, no medications presently
approved for NAFLD. The primary therapeutic intervention in
NAFLD, same as in other MetS is gained from lifestyle improvement,
promoting equitable low-energy diet, together with promoting
physical activity. So, these are prime remunerative approaches
for this condition [12,13]. Lifestyle moderation has improved
the metabolism syndrome features; however, more effort needs
to be made in addressing various MetS components [14]. Thus,
to provide better understanding that will help different players
involved in management of MetS disorders. This review discusses
various mechanisms of developing NAFLD and its interplay with
other metabolic disorders, while clarifying beneficial mechanisms
for improvement.
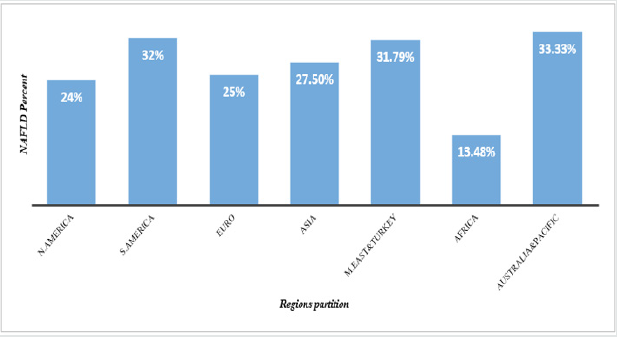
Figure 1: NAFLD Prevalence in global remarkable regions.
N. America; North America, S. America; South America, Euro; Europe, M. East; Middle East.
Prevalence of NAFLD
The NAFLD prevalence increases quickly in every region in the
world as a masked epidemic. The worldly estimation of NAFLD
prevalence in general population has surpassed 25% [10] (Figure
2).
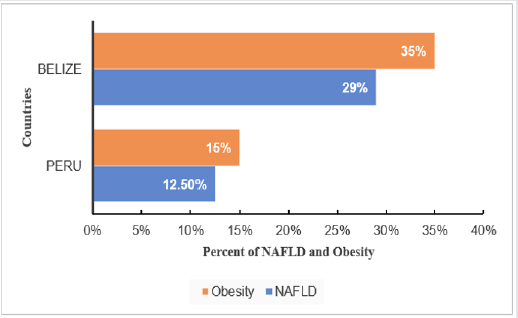
In region of South and Central America, NAFLD prevalence
varies depending on obesity rate, where Belize has the highest
obesity prevalence of 35% with NAFLD 29% and Peru has the
lowest obesity prevalence of 15% with NAFLD 12.5% [10]. The Occurrence of obesity aggravate NAFLD prevalence. In addition,
NAFLD is sharply elevating worldwide, coincident with the
augmented prevalence of obesity. Currently NAFLD has become the
major chronic liver disease; NAFLD prevalence in adult population
of developed countries is approximately 30% [9]. NAFLD has also
become a considerable liver disease in children in response to the
elevation of childhood obesity prevalence. Obesity may initiate
production of excess ROS and systemic oxidative stress [15] which
result in protein and lipid oxidation [16] (Figure 3).
Figure 3: Influence of Genetic PNPLA3 on NAFLD in USA.
H. Puerto Rican; Hispanic from Puerto Rican origin, H. Dominican; Hispanic from Dominican origin, H. Mexican; Hispanic
from Mexican origin.
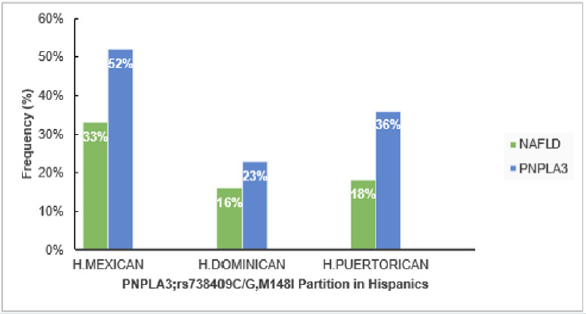
The study from sub-region of USA that compared NAFLD in Hispanic from different origins, showed the greatest NAFLD prevalence in Hispanics from Mexican origin (33%) than those from Caribbean origin (Puerto Rica (18%), Dominic Republic (16%)), (P<0.01). The greater NAFLD prevalence remained in Hispanics of Mexicans than Dominican or Puerto Rican origin, even after regulating other factors that discovered to contribute in NAFLD; such as insulin resistance, levels of triglyceride and C-reactive protein, hypertension, serum level of high-density lipoprotein, waist circumference, body mass index (BMI), sex and age [17]. Prevalence of NAFLD might be explained by elevated polymorphism prevalence in the gene encoding patatin-like phospholipase domain-containing 3 (PNPLA3; rs738409 C/G, M148I); especially in Hispanics where it accounts (49%), compared to Non-Hispanics whites (23%) and African-American (17%) [18,19]. Moreover, PNPLA3; rs738409 counts 52% in Hispanics Mexican, 23% in Dominicans [20] (Eric et al., 2019) and 36% in Hispanics Puerto Rican [21]. According to a genome-wide association study [19] elucidated that a non-synonymous sequence variation (rs738409) in PNPLA3 that replaces methionine for isoleucine at residue 148 (I148M) correlates with disproportion in liver lipid profile and the possibility of generating NAFLD. Thus, higher PNPLA3; rs738409 C/G, M148I in Hispanics suggested to take part in fueling NAFLD high prevalence [22] (Figure 4).
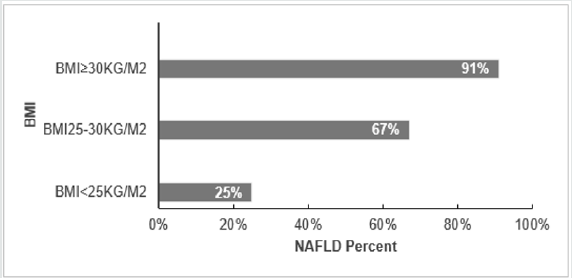
BMI correlates with NAFLD, and it has been revealed as a factor that elevates the probability of emerging NAFLD. In years, 1980-2008, the BMI-mean augmented worldwide by 0.4 kg/ m2 for males and by 0.5 kg/ m2 for females per decade. This coincides with obesity prevalence that increased simultaneously from 4.8 to 9.8% for men and from 7.9 to 13.8% for Women [23]. This factor was investigated in the region of European Union and it’s clear that similarly to other regions, in Europe the higher risk of acquiring NAFLD is elevated coincidently with the increases of BMI, 25% (BMI<25kg/ m2), 67% (BMI 25-30 kg/ m2) and 91% (BMI ≥30 kg/ m2) [24]. Together with other risk factors (obesity or T2DM) which more likely associate to sedentary life, are increasing in Europe fueling the NALFD prevalence and further the impairments such as cirrhosis and HCC [25].
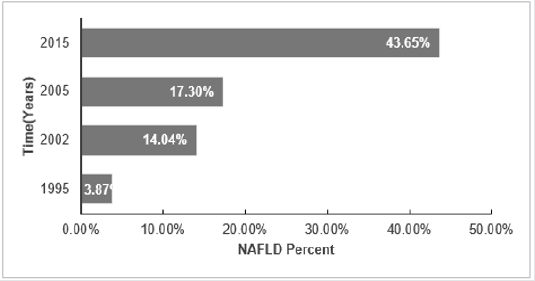
The NAFLD prevalence among adults of sub- region Shanghai was considered to show high growing trend of NAFLD with time, it’s obvious that NAFLD prevalence is elevating with time [26]. The research showed diversity of NAFLD prevalence in different subregions of Asian countries, in Taiwan (15%-27%), Korea (24%- 40%), and Japan (9%-18%) [27]. Additionally, prevalence in India has increased from 28% in 2015 to 31% in 2016 in the rural region of Haryana [28]. Timely elevation of Fatty Liver was also revealed in different sub-regions of China [29]. Shanghai with high rate in development that goes together with life behavioral changing; the incremental in NAFLD is seen to increase sharply in adults in recent years. Findings supported by [30-33], shown in Figure 5.
NAFLD Pathogenesis
Numerous researches have reported that metabolic
impairment or disturbance is the basic abnormality in NAFLD [34].
At the center of this discovered metabolic abnormality there is
insulin resistance, that also known among the initiators of NAFLD.
Thereafter, by provoking oxidative stress, fatty liver may evoke
inflammation and hepatocyte injury which may cause the disease
to tend to NASH and cirrhosis. The overload of triglycerides (TG)
as fat droplets in hepatocytes cytoplasm was reported as the main
event of NAFLD, which is a precondition for the succeeding NASH,
as it was shown by liver biopsy that 5%-10% of hepatocytes have
fat droplets [35]. Increased moving of both TG and free fatty acids
(FFA) to the liver, reduced hepatic using of FFA, decrease in export
of TG from liver, disturbed beta-oxidation of FFA in hepatocytes
result in stockpiling of TG within hepatocytes cytoplasm [34,36,37].
Another main stimulus for liver de novo fatty acid synthesis, is the
surplus carbohydrate from either dietary origins or hepatic de novo
gluconeogenesis [36,37].
Obesity can be defined as a chronic low-grade inflammatory
condition. There are cytokines related to obesity, such as
interleukin-6 (IL-6), leptin, adiponectin, and tumor necrosis factoralpha
(TNF-α) that play a remarkable role in NAFLD evolution.
Research findings reported that adipose tissue may be the inducer of
inflammatory mediators and adipokines, such as pro-inflammatory
ones (IL-6, TNF-α, and leptin) and anti-inflammatory (adiponectin)
effects [38]. Even though, these hormones and cytokines may
ordinally work in balance, the homeostasis is disturbed in NASH
that result in increased TNF-α and reduced adiponectin levels.
Several mechanisms that likely involve in hepatocellular injury
in setting of NAFLD, many of them produce the ROS. A liver with
surplus fat can be more prone to stressors, such as adipokines,
reactive ROS, and cytokines than normal liver [39,40].
In the study done by Yang and colleagues, obese mice with fatty
liver cleared endotoxins less than nonobese controls [38]. Factors
that perform key functions in the evolution of NASH from simple
steatosis remain unclear [41-43]. Some known possible ways are
oxidative stress through increasing ROS and reduced antioxidants,
lipid peroxidation, reactive metabolites, 4-hydroxynonenal and
malondialdehyde, adipose tissue products. Other discovered ways
are Fas-ligand, transforming growth factors-β1, respiratory chain
deficiency along with mitochondrial dysfunction, and intestinal
microbiota by augmented intestinal permeability, elevated energy
gaining from diet, intestinal leak, bacterial lipopolysaccharides
(LPS), TNF-α, and endotoxins [44].
Hallmarks in NAFLD
NAFLD and oxidative stress
Oxidative stress increases the loss of structure and function of healthy cells, DNA and also damage of important macromolecules. These conditions are the main roots for chronic diseases such as cancer, stroke, cardiovascular impairment including diabetes [45]. Oxidative stress has been found to disrupt insulin signaling process, which result in insulin resistance in cell [46]. Yet, impaired insulin signaling mechanism remains unclear [47] (Rains & Jain, 2011). Besides, Elevation of oxidative stress is generally a considerable factor in degenerative diseases, including chronic fatigue neurodegenerative diseases. Moreover, oxidative stress can be suggested to mediates the transition from simple steatosis to steatohepatitis and, disruption of metabolic balance. Furthest, the above cited impairments are the crucial features that lead to steatohepatitis in lipid-laden hepatocytes [48]. Oxidative stress arises due to the lower antioxidant capacity and/or overproduction of the ROS [47]. Various mechanisms by which these two scenarios initiate oxidative stress in organism are described in the following paragraphs.
Several factors initiate oxidative stress in diabetes. The main
origin of oxidative stress is mitochondria. Mitochondria use around
98% inhaled oxygen, from which 0.2-2% produce ROS [47,49]. A
part of the used oxygen is reduced to water, and the left oxygen
converted to oxygen free radicals through oxidative metabolism of
mitochondria [50]. Oxygen is required for human life, even though,
it may damage and kill the cells when it produces ROS [51]. The
ROS are engendered by the reduction of molecular oxygen or from
oxidation of water to give products such as superoxide anion,
hydroxyl radical, hydrogen peroxide [47]. The hyperglycemia
promotes low density lipoprotein (LDL) peroxidation and followed
by the production of free radicals [52,53]. The other significant
factor in the production of free radicals is glucose oxidation. In
its enadiol form, glucose become oxidized in a transition-metal
dependent reaction to give enadiol radical anion transformed
into reactive radicals [54]. Other mechanism of oxidative stress
in diabetes is the engendering of advanced glycated end products
(AGEs) [55]. AGEs resulted from the covalent binding of the ketone
or aldehyde groups of reducing sugars, in their way to free the
amino groups of proteins [47].
Alternatively, Antioxidant may be defined as a substance that
delay or inhibit the oxidation of substrate, this scenario comprises
many mechanisms pointed out in oxidative stress production
pathways. There exist various exogenous and endogenous
components which can perform a considerable function in
antioxidant defense and help in prevention of oxidative stress in
organism including Catalase (CAT), glutathione peroxidase (GPx),
superoxide dismutase (SOD) [56]. The alteration of endogenous
antioxidant defense comes in case of hyperglycemia. In diabetes,
both decreases and increases in the activities of major antioxidant
enzymes like CAT, SOD, Glutathione reductase (GR), GPx have
been noticed [57]. Studies have shown that in pancreatic islets
8-hydroxy-2-deoxyguanosine (8-OH-dG) and 4-hydroxynonenal (4-
HNE) levels augmented which support that hyperglycemia might
be a key inducer of oxidative stress in the β-cell and oxidative
stress induced by glucose [58]. The β-cells of pancreatic islets are
vulnerable to the genesis of ROS and the reduction of antioxidant
enzymes activities [59]. In Goto-Kakizaki (GK)-rats, the levels of
8-OH-dG and 4-HNE were high in β-cells of pancreatic islets [60].
Many reports have elucidated that the increasing of peroxide
content in tissues, plasma, and red blood cells of animals that have
chemically induced diabetes [61,62].
NAFLD and Type 2 Diabetes
The apparition of NAFLD exposes to possibility of T2D and in such way exacerbates its complications [63-66]. Multiple factors such as inflammatory adipocytokines and free fatty acids that efficaciously engage in initiation of insulin resistance and NAFLD, are known to be originated from inflamed and inflated visceral adipose tissue [67]. Liver is the prime body organ targeted by accumulation of ectopic fat, and superfluous free fatty acid (FFA) flow into the liver support insulin resistance through initiating NF-kB induction, lysosomal instability, and TNFα activation [68] , cAMP/ PKA pathway [69], or by activation of IL-18 production and NLRP3-mediated IL-1β [70]. The activation of c-Jun N-terminal kinase (JNK) and protein kinase Cε (PKCε) are known to be two ways through which intermediate of liver fat synthesis (Diacylglycerol (DAG)) inhibits signaling of liver insulin [3]. The hepatocytes initiate a redemptive procedure by augmenting mitochondrial β-oxidation to stop FFA, as consequence excess lipid will then hinder capacity of mitochondria antioxidant. Furthest, these will promote insulin resistance that lead to leakage of mitochondria and oxidative stress [71].
Table 1: Various factors expressed in liver steatosis condition that involve advanced complications.
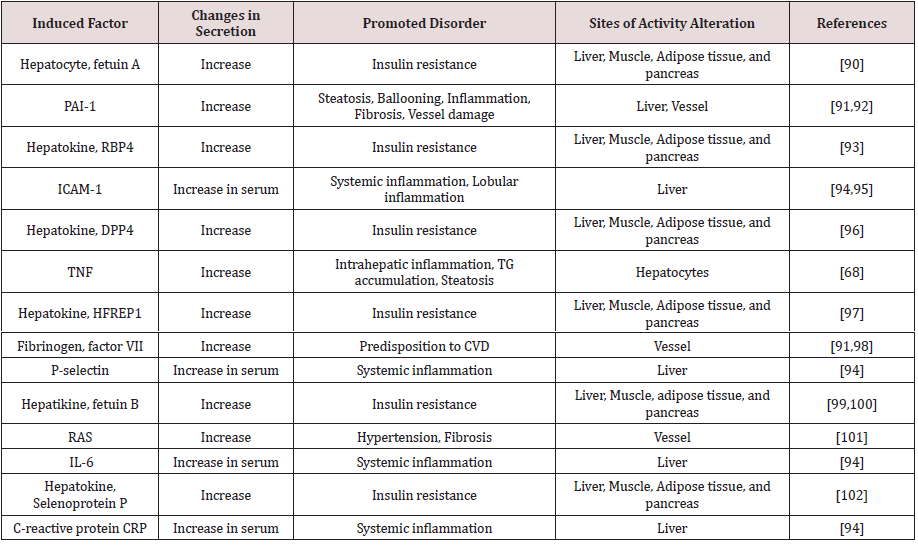
In hepatic insulin resistance, two pathways are proposed to
stimulate de novo lipogenesis, either by glucose, via carbohydrate
response element-binding protein (ChREBP) [72] or by insulin
via sterol regulatory element-binding protein 1c (SREBP-1c) [73].
In addition, insulin resistance obviously known to be induced
by the alteration of metabolisms in muscle, liver, pancreas, and
adipose tissue that might be initiated from hepatokines. Recently, a
number of studies substantiated that secretion of hepatokines with
diabetogenic properties become disturbed during liver steatosis
condition, See Table 1. Liver steatosis reduces high-density
lipoprotein cholesterol (HDL), and increases triglycerides (TG) and
low-density lipoprotein cholesterol (LDL). As result, these changes
favor the atherogenic dyslipidemia [74]. Likewise, liver steatosis
stimulates inflammatory factors and disturbs agents involved in
blood coagulation and circulation, which also predispose to CVD
risk See Table 1. All these scoped out mechanisms related to NAFLD
pathogenesis are proposed to augment the risk of microvascular
and macrovascular diabetic complication. Notably, improvement of
NAFLD has been revealed to be a manner of modifying probability
of generating diabetes [75].
Existing of T2D promote the possibility of NASH generation
by two- to three-fold (Portillo-Sanchez et al., 2015). In T2D
patient’s lipogenesis is high in liver [72,73], accompanying fatty
acid oxidation and production of triglyceride. On the other hand,
very low-density lipoprotein cholesterol (VLDL) will decrease
[76], in this manner, the two incidence NAFLD and T2D often
coexist. Primarily, sympathetic fat liver accumulation arises as
a defense response to lipotoxicity of free fatty acids that induce
metabolic stress [77]. Even though, continual liver free fatty acid
influx augment in background, resulting hepatic intracellular
triglycerides activate different inflammatory pathways [48].
There are still several outrages in NAFLD patients with T2DM that
through different mechanisms initiate the proceeding of NAFLD
to NASH, cirrhosis, and HCC [78]. FFA like, ceramides, cholesterol,
palmitic acid, and lysophosphatidylchloline; their extra influx in
liver of T2D patients, directly lead to lipotoxicity and finally give
rise to liver inflammation and fibrosis [79,80]. Metabolism of
excess FFAs and oxidation in liver generate oxidative stress [81],
which in combination with endoplasmic reticulum (ER) stress [82]
lead to hepatocellular injury and apoptosis. Moreover, extra hepatic
FFAs, the dissemination of inflammatory mediators (like TNFα, IL-
6, and MCP-1) from disordered adipose tissue [80], and endotoxins
emanated from gut [83], are obviously mentioned in diabetic
patients with NAFLD. As result, these will activate Kupffer cells in
liver [84] and release hepatic inflammatory mediators (IL-1β, IL-6,
TNFα) that will consequently fuel inflammation and liver damage
[85]. The generated hepatocellular damage actuates necrotic and
apoptotic hepatocyte death pathways [86], so the continuation
of this mechanism finally activates liver stellate cells, deposits of
collagen, and liver fibrosis [87]. In another way, with no influence
of liver stellate cells activity, liver fibrosis can be generated from
insulin resistance by mediating lysyl oxidase-like 2 (Loxl2) [88].
In parallel, oxidative stress, liver inflammation, insulin resistance,
ER stress, and hepatocyte death may also induce the regenerative
procedure through a lines of growth factors that activate a number
of oncogenic signaling pathways, such as JAK/STAT, PI3K/PTEN/
Akt, mTOR, NF-kB, NRF-1, and 4HN. And these mechanisms
disclosed above contribute to the generation of HCC [89].
NAFLD and Blood Vessels Impairment
Liver is regarded a central organ, that favor its interrelation with various system like cardiovascular and others such as gut, visceral and subcutaneous adipose tissue, and muscle tissues [103]. Circumstances like long-term lipid and glucose metabolism disturbance, oxidative stress, insulin resistance can promote vascular endothelial dysfunction, which is related to endothelial cell damage originated from biological and physical changes including hypoxia and ischemia, hemodynamics, and lipid deposition [104,105]. The lipid overaccumulation and hypercholesterolemia induced by NAFLD can so far, result in endothelial dysfunction subsequent to vascular diseases (VD). Undetermined endothelial function is an early stage in the acquisition of atherosclerosis, ahead of development of plaque inflammation or fatty streaks [106] and hence crucial in CVD development. Postprandial lipid status is consistent with atherogenic form through augmented chylomicron remnants, small quantity of HDL particles and more LDL [107] (Roche and Gibney, 2000). In case of NAFLD patients, the postprandial pathway is hallmarked by enlarged VLDL particles and excess levels of triglyceride-rich [50,108]. Suggested mechanisms through which NAFLD intervene in CVD remain complex and heterogenous. As far as NAFLD obviously known as part of systemic disease and expression of MetS in liver its intercorrelation with CVD could be drawn from the fact that, liver plays an important function in lipid and glucose homeostasis, and hence, is the center of cardiometabolic disease. One of the beginning points is possibly a disparity in calorie-intake and expenditure, overfilling the adipose tissue capacity of storage resulting to the buildup of ectopic fat, involving hepatic fat [42]. Liver enzymes elevation are correlated to stroke [109]. NAFLD Patients expose endothelial malfunction of conducting vessels, along with microvasculature [110]. Arterial stiffness known as a well-recognized marker of CVD anteceding arterial hypertension, and NAFLD remain independently allied to rising of vascular stiffness [110]. Discrepancy of living condition, glycaemic control and body weight may substantially influence disease progression.
A make for the effective carotid plaque burden/generalized atherosclerotic burden known as carotid intimal media thickness (cIMT) interplays with NAFLD [111]. In case of cirrhosis, intrahepatic and mesenterial endothelial irregularity are encountered [112]. Same as in NAFLD, intrahepatic dysfunction appeared [113] yet, interestingly, no inflammation or fibrosis reported [114,115]. So, this is implicated as an early phenomenon that proposed to lead to disease progression. Even though is more pronounced in NASH, Endothelial dysfunction of systemic circulation has been also found in NAFLD [116]. An endogenous antagonist of nitric oxide synthase (NOS) known as Asymmetric dimethyl arginine (ADMA) was discovered to be positively allied to CVD. Reduced breakdown, in which liver perform a vital function [117], is suggested to result in elevated ADMA levels [77]. Moreover, NAFLD patients show elevated quantity of circulating ADMA, a coalition ceasing after the reparation for metabolic risk factors [117]. Other numerous markers involved in endothelial dysfunction (like, endocan) have been also found to elevate in NAFLD [118] \. An undisturbed endothelial monolayer is fundamental for normal vessel wall performing. Ruination of this layer performs a role in atherogenesis, that is marked by endothelial microparticles (EMPs) elevation, showing disruption of endothelial. Apart, the endothelial progenitor cells (EPCs), reported to indicate endothelial repairmen, their circulating levels decreased, and their adhesive activity also weakened in NAFLD [119].
Liver microvasculature manifest a remarkable modification in NAFLD condition, these are: deformation of the sinusoidal pattern, sinusoids compress by fat-burdened hepatocytes and fenestrae ruination [120]. And these distortions appear due to the generation of fibrosis and inflammation as a sign of preliminary phenomenon [114]. These disproportions in structure are known to take part in accelerating portal pressure observed in non-cirrhotic NAFLD, in humans and animals [114]. In arterial stiffness condition that increases relating to NAFLD and CVD, there is alteration in structure of ‘media’ in large arteries: its crosslinking and collagen content increases, contrarily elastin fibres decrease, so as to get fractioned [121]. Quantities of metalloproteinases, same as serum elastase are connected with arterial stiffness [121]. Interestingly, in NAFLD this arterial stiffness was found to be elevated [122], others made a proposition of a potential activity of TGF-β [123]. The role of preventing the formation of atherosclerosis done by laminar flow on vascular endothelium known to be induced by Nrf2 activation (Kim et al. 2012). Furthest, Nrf2 has been yet reported to extenuate neointimal hyperplasia originated from vascular injury [124].
Non-Coding Rnas Improve NAFLD
Non-Coding RNAs (ncRNAs) is a set of molecules that is regularly incorporated in NAFLD pathogenesis. ncRNAs typically shows high cellular or tissue specificity, allowing them great potential for predicting disease progression. In case of NAFLD as well as in other diseases ncRNAs may have a critical role as appropriate biomarkers and good indicators in assessing the severity of disease [125]. Most examined non-coding RNAs are miRNAs. Times ago, panels encompassing several serum miRNAs and other biochemical indicators, unveiled miRNAs to have high diagnostic indices for NAFLD and a higher predicted NASH potential compared to biomarkers [126,127]. In addition, miRNAs have been discovered to intervene in lipid metabolism, inflammation, insulin resistance, fibrosis, as well as HCC generation. Knowing their correlation with severity of disease, miRNAs can be biomarkers used in early noninvasive diagnosis and evaluation of NAFLD severity. Sensitive biomarker for early detection is accessed through assessing miRNAs serum levels [125]. Therefore, some critical points and pathways are scoped out in the following paragraphs.
First point investigates the role of miR-155, this has shown the ability to regulate some proteins and cytokines obviously known to participate in NAFLD progression, as cleared in following discussion. The Cytokines TNFα, IL-6 have been suggested to perform a greater role in NAFLD pathogenesis [128], as their elevation and secretion have been discovered to be increasing in serum of NASH patient (Kassel et al., 2010). Again, Park et al. unveiled that pro-inflammatory cytokines IL-6 and TNFα are crucial for the progression from steatosis to steatohepatitis in obese mice, and that absence of IL-6 or TNFR1( receptor that bind TNFα) decreased lipid accumulation in liver, as well as reduction of macrophages and neutrophils influx in high fat fed diet mice [129]. Beyond this, in their absence, levels of reactive oxygen were also lowered, together with the AKT, mTOR and COX2 proteins. The findings of Qu et al. have reported similar suppression of IL-6 and IL-1b in response to miR-155 or miR-21 inhibitors, showing that these inflammatory factors are regulated positively by miR-21 or miR-155. In addition, obesity and NAFLD in another way can develop in response to elevated white adipose tissue mass corresponding to miR-155 deficiency/downregulation. Furthest, hepatic steatosis augmented in miR-155 mice fed with HFD for 6 months [130].
Second point investigates the role of miR-22, for its elucidated
potentiality in revealing different degrees of liver abnormalities in
NAFLD, as cleared in this paragraph. The research findings revealed
that the serum level of miR-122 in mice with a methionine-choline
deficiency (MCD) diet has augmented 40-fold, overstepping
serum alanine aminotransferase (ALT) (4.8-fold) and aspartate
aminotransferase (AST) (3.3-fold) [131]. Additionally, it was
explicated that increased levels of serum miR-122 occurred in
NAFLD rats even without elevated ALT augmentation [132]. Hence,
the sensitivity of miR-122 is better than the one of cytokeratin (CK)-
18, ALT, or AST while detecting NASH and predicting liver fibrosis in
patients with NAFLD [133].
Another complication that commonly involve in pathogenesis of
several diseases is the abnormal cell proliferation. So, the inhibition
of this pathological process has been clarified as critical way in
treating precited disease [134], through targeting miRNA, lncRNA,
or ceRNA crosstalk. In case of elevated quantity of PPARα, its target
gene carnitine palmitoyltransferase 2 (CPT2) and the acyl-CoA binding domain containing 3 (ACBD3) or the solute carrier family
27A (SLC27A) become activated, and the steatosis is lowered [134].
Therefore, scircRNA/miR-34a/PPARα Pathway is suggested to be
incorporated in progression of NAFLD. Moreover, the critical role
of noncoding RNAs in NAFLD was investigated in a number of
studies which explained that both circRNA 0046367 and circRNA
0046366 the endogenous regulators of miR-34a [134, 135] block
miRNA/mRNA cooperation with miRNA response element (MRE)
and lastly can extinct inhibitory effect on PPARα. These enabled
to propose that dysregulation mechanism of circRNA 0046366 or
circRNA 0046366/miR-34a/PPARα signaling pathway may be a
novel mechanism that lead to hepatic steatosis [134].
Conclusion
NAFLD is etiologically correlated with systemic distortion of various metabolisms, of which their impairment result in multiple disorders such as: insulin resistance, hyperglycemia, hyperlipidemia and other complications correlated with obesity and diabetes. Insulin resistance is illustrated like a central root that likely initiates the coexistence of diabetes and NAFLD. Moreover, mitochondria hyperactivity and shortage in antioxidants initiate liver oxidative stress. That induce inflammation which aggravate the liver cell damage by accelerating the progression from simple steatosis to NASH, that progress to other irreversible forms of NAFLD. Besides, cytokines related to obesity, such as interleukin-6 (IL-6), leptin, adiponectin, and tumor necrosis factor-alpha (TNF-α) work in harmony, but their homeostasis is disturbed in NASH, that also worsen NAFLD. The prevalence of NAFLD elevated the risks of acquiring other diseases either related to Insulin resistance, as co-inducer (such as Type 2 diabetes) or from subsequent damages (such as cardiovascular diseases, from vessels damage). The management of NAFLD rely on early detection and severity quantification, the noncoding RNAs have shown these abilities with high sensitivity. Obesity and NAFLD can also develop in response to elevated white adipose tissue mass, derived from miR-155 deficiency/downregulation. These facts allow us to suggest also that noncoding gene can play role in handling the disease. Moreover, also many other different mechanisms involved in developing NAFLD, and interconnecting pathways with other metabolism complications (such as diabetes, obesity and cardiovascular diseases) are encompassed in this study. However, currently no specific medication for NAFLD, future researches can base on advancing analysis that amalgamate findings on NAFLD in its complexity and its interrelation, like current study, to further in management of NAFLD and in case diverse disorders involved to aggravate condition.
Acknowledgement
This study was supported by National Natural Science Foundation of China (30725045).
References
- Marrero JA, Robert J Fontana, Grace L Su, Hari S Conjeevaram, Dawn M Emick, et al. (2002) NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 36(6): 1349-1354.
- Malaguarnera L, Roberto Madedduc, Elisabetta Palioa, Nicolò Arenac, Mariano Malaguarnerab, et al. (2005) Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. Journal of hepatology 42(4): 585-591.
- Jornayvaz FR, GI Shulman (2012) Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell metabolism 15(5): 574-584.
- Boyajian TS, Harold A McAlister, Gerard van Belle, Douglas R Gies, Theo A ten Brummelaar, et al. (2012) Stellar diameters and temperatures I Main-sequence A F and G stars. The Astrophysical Journal 746(1): 101.
- Vanni E, Elisabetta Bugianesia, Anna Kotronenb, Samuele De Minicisd, Hannele Yki-Järvinenb, et al. (2010) From the metabolic syndrome to NAFLD or vice versa?. Digestive and liver Disease 42(5): 320-330.
- Reaven G (2002) Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation 106(3): 286-288.
- Williams J (2014) Understanding poststructuralism. Routledge, UK.
- Rinella ME, AJ Sanyal (2016) Management of NAFLD: a stage-based approach. Nature reviews Gastroenterology & hepatology 13(4): 196-205.
- Marchesini G, Elisabetta Bugianesi, Gabriele Forlani, Fernanda Cerrelli, Marco Lenzi, et al. (2003) Nonalcoholic fatty liver steatohepatitis and the metabolic syndrome. Hepatology 37(4): 917-923.
- Younossi Z, L Henry (2016) Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 150(8): 1778-1785.
- Duseja A, Shivaram P Singh, Vivek A Saraswat, Subrat K Acharya, Yogesh K Chawla, et al. (2015) Non-alcoholic fatty liver disease and metabolic syndrome-position paper of the Indian National Association for the Study of the Liver Endocrine Society of India. Indian College of Cardiology and Indian Society of Gastroenterology. Journal of clinical and experimental hepatology 5(1): 51-68.
- Blond E, Emmanuel Disse, Charlotte Cuerq, Jocelyne Drai, Pierre-Jean Valette, et al. (2017) EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: do they lead to over-referral? Diabetologia 60(7): 1218-1222.
- Oseini AM, AJ Sanyal (2017) Therapies in non‐alcoholic steatohepatitis (NASH). Liver International 37(s1): 97-103.
- Graf BL, Raskin I, Cefalu WT, Ribnicky DM (2010) Plant-derived therapeutics for the treatment of metabolic syndrome. Current opinion in investigational drugs 11(10): 1107-1115.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation 114(12): 1752-1761.
- Matsuda M, Shimomura I (2014) Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Reviews in Endocrine and Metabolic Disorders 15(1): 1-10.
- Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP (2016) Nonalcoholic fatty liver disease in Latinos. Clinical Gastroenterology and Hepatology 14(1): 5-12.
- Guerrero R, Vega GL, Grundy SM, Browning JD (2009) Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49(3): 791-801.
- Romeo S, Julia Kozlitina, Chao Xing, Alexander Pertsemlidis, David Cox, et al. (2008) Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics 40(12): 1461-1465.
- Kallwitz ER, Tayo BO, Kuniholm MH, Cai J, Daviglus M, et al. (2019) American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clinical Gastroenterology and Hepatology 17(11): 2301-2309.
- Edelman D, Kalia H, Delio M, Alani M, Krishnamurthy K, et al. (2015) Genetic analysis of nonalcoholic fatty liver disease within a Caribbean–Hispanic population. Molecular genetics & genomic medicine 3(6): 558-569.
- Younossi ZM, Stepanova M, Rafiq N, Henry L, Loomba R, et al. (2017) Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatology communications 1(5): 421-428.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, et al. (2011)National regional and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. The lancet 377(9765): 557-567.
- Bedogni G, Miglioli L, Masutti F, Castiglione A, Crocè LS, et al.(2007)Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology 46(5): 1387-1391.
- Seto W-K, M-F Yuen (2016) Nonalcoholic fatty liver disease in Asia: emerging perspectives. Journal of gastroenterology 52(2): 164-174.
- Mahady SE, J George (2018) Predicting the future burden of NAFLD and NASH. Journal of hepatology 69(4): 774-775.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease-meta‐analytic assessment of prevalence incidence and outcomes. Hepatology 64(1): 73-84.
- Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, et al. (2017) Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology 65(1): 54-64.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY (2014)The global burden of liver disease: the major impact of China. Hepatology 60(6): 2099-2108.
- Fan JG, Fen Li, Xiao-Bo Cai, Yong-De Peng, Qing-Hong Ao, et al. (2007) Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. Journal of gastroenterology and hepatology 22(7): 1086-1091.
- Fan JG, Zhu J, Li XJ, Chen L, Li L, et al. (2005) Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. Journal of hepatology 43(3): 508-514.
- Fan JG (2013) Epidemiology of alcoholic and nonalcoholic fatty liver disease in C hina. Journal of gastroenterology and hepatology 28(1): 11-17.
- Yang Z, Yan C, Liu G, Niu Y, Zang W,et al. (2016) Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: a cross-sectional analysis. Scientific reports 6(1):1-8.
- Neuschwander-Tetri, Brent A (2007) Fatty liver and the metabolic syndrome.Current opinion in gastroenterology 23(2): 193-198.
- Miyake T, Kumagi T, Hirooka M, Furukawa S, Hiasa Y, et al. (2015) Significance of exercise in nonalcoholic fatty liver disease in men: a community-based large cross-sectional study. Journal of gastroenterology 50(2): 230-237.
- Browning JD, Horton JD (2004) Molecular mediators of hepatic steatosis and liver injury. The Journal of clinical investigation 114(2): 147-152.
- Crespo J, Cayón A, Mayorga M, Escalante JC, Fernández-Gil P, et al. (2001) Gene expression of tumor necrosis factor α and TNF‐receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34(6): 1158-1163.
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM, et al. (1997) Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proceedings of the National Academy of Sciences 94(6): 2557-2562.
- Basaranoglu M, Kayacetin S, Yilmaz N, Kayacetin E, Tarcin O, et al. (2010) Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World journal of gastroenterology 16(18): 2223-2226.
- Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA, et al. (2008) Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. American Journal of Physiology-Gastrointestinal and Liver Physiology 295(5): G987-G995.
- Basaranoglu M, Nesrin Turhan, Abdullah Sonsuz, Gökcen Basaranoglu, et al. (2011) Mallory-Denk Bodies in chronic hepatitis. World Journal of Gastroenterology 17(17): 2172-2177.
- Heilbronn L, Smith S, Ravussin E (2004) Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. International journal of obesity 28(4): S12-S21.
- Neuschwander-Tetri, Brent A, David A. Ford, Sahaja Acharya, George Gilkey, et al. (2012) Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools. Lipids 47(10): 941-950.
- Başaranoğlu M, Örmeci N (2014) Nonalcoholic fatty liver disease: diagnosis, pathogenesis, and management. Turk J Gastroenterol 25(2): 127-132.
- Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344(8924): 721-724.
- Wright Jr E, Scism‐Bacon BL, LC Glass (2006) Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. International journal of clinical practice 60(3): 308-314.
- Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radical Biology and Medicine 50(5): 567-575.
- Sharma M, Mitnala S, Vishnubhotla RK, Mukherjee R, Reddy DN, et al. (2015) The riddle of nonalcoholic fatty liver disease: progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Journal of clinical and experimental hepatology 5(2): 147-158.
- Duchen MR (2004 ) Roles of mitochondria in health and disease. Diabetes 53(1): S96-S102.
- Moussa S (2008) Oxidative stress in diabetes mellitus. Romanian J biophys 18(3): 225-236.
- Weseler AR, Bast A (2010) Oxidative stress and vascular function: implications for pharmacologic treatments. Current hypertension reports 12(3): 154-161.
- Kawamura M Heineckeand J W, Chait A (1994) Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. The Journal of Clinical Investigation 94(2): 771-778.
- Tsai EC, Hirsch IB, Brunzell JD, Chait A (1994) Reduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDM. Diabetes 43(8): 1010-1014.
- Jiang ZY, Woollard AC, Wolff SP (1990) Hydrogen peroxide production during experimental protein glycation. FEBS letters 268(1): 69-71.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865): 813-820.
- Somogyi A, Rosta K, Pusztai P, Tulassay Z, Nagy G, et al. (2007) Antioxidant measurements. Physiological measurement 28(4): R41-R55.
- Godin DV, Wohaieb SA, Garnett ME, Goumeniouk AD (1988) Antioxidant enzyme alterations in experimental and clinical diabetes. Molecular and cellular biochemistry 84(2): 223-231.
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP, et al. (1999) Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proceedings of the National Academy of Sciences 96(19): 10857-10862.
- Grankvist K, Marklund SL, Täljedal IB (1981) CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochemical Journal 199(2): 393-398.
- IharaY, Toyokuni S, Uchida K, Odaka H, Tanaka T, et al. (1999) Hyperglycemia causes Oxidative Stress in Pancreatic B-Cells of GK Rats, a Model of Type 2 Diabetes. Diabetes 48(4): 927-932.
- Armstrong D, Al-Awadi F (1991) Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Radic Biol Med 11(4): 433-436.
- Rungby J, Flyvbjerg A, Andersen HB, Nyborg K (1992) Lipid peroxidation in early experimental diabetes in rats: effects of diabetes and insulin. Acta Endocrinol (Copenh) 126(5): 378-380.
- Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M (2007) Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes care 30(11): 2940-2944.
- Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, et al. (2008) Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol 3(5): 1296-1300.
- Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK (2009) NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 104(4): 861-867.
- Ryoo JH, Choi JM, Moon SY, Suh YJ, Shin JY, et al. (2013) The clinical availability of non alcoholic fatty liver disease as an early predictor of the metabolic syndrome in Korean men: 5-year's prospective cohort study. Atherosclerosis 227(2): 398-403.
- Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132(6): 2169-2180.
- Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, et al. (2004) Free fatty acids promote hepatic lipotoxicity by stimulating TNF‐α expression via a lysosomal pathway. Hepatology 40(1): 185-194.
- Ke B, Zhao Z, Ye X, Gao Z, Manganiello V, et al. (2015) Inactivation of NF-κB p65 (RelA) in liver improves insulin sensitivity and inhibits cAMP/PKA pathway. Diabetes 64(10): 3355-3362.
- Ralston JC, Lyons CL, Kennedy EB, Kirwan AM, Roche HM (2017) Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu Rev Nutr 37: 77-102.
- Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, et al. (2015) Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21(5): 739-746.
- Linden AG, Li S, Choi HY, Fang F, Fukasawa M, et al. (2018) Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J Lipid Res 59(3): 475-487.
- Tian J, Goldstein JL, Brown MS (2016) Insulin induction of SREBP-1c in rodent liver requires LXRα-C/EBPβ complex. Proc Natl Acad Sci 113(29): 8182-8187.
- Amor AJ, Pinyol M, Solà E, Catalan M, Cofán M, et al. (2017) Relationship between noninvasive scores of nonalcoholic fatty liver disease and nuclear magnetic resonance lipoprotein abnormalities: A focus on atherogenic dyslipidemia. J Clin Lipidol 11(2): 551-561.
- Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H (2015) Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care 38(9): 1673-1679.
- Kamagate A, Dong HH (2008) FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 7(20): 3162-3170.
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, et al. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115(5): 1343-1351.
- Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8): 1038-1048.
- Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, et al. (2014) Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 59(1): 154-169.
- Marra F, Svegliati-Baroni G (2018) Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 68(2): 280-295.
- Paradies G, Paradies V, Ruggiero FM, Petrosillo G (2014) Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol 20(39): 14205-14218.
- Muraki Y, Makita Y, Yamasaki M, Amano Y, Matsuo T (2017) Elevation of liver endoplasmic reticulum stress in a modified choline-deficient l-amino acid-defined diet-fed non-alcoholic steatohepatitis mouse model. Biochem Biophys Res Commun 486(3): 632-638.
- Carnevale R, Pastori D, Nocella C, Cammisotto V, Baratta F, et al. (2017) Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr Metab Cardiovasc Dis 27(10): 890-895.
- Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, et al. (2019) The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 16(3): 145-159.
- Yu Y, Liu Y, An W, Song J, Zhang Y, et al. (2019) STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest 129(2): 546-555.
- Luedde T, Kaplowitz N, Schwabe RF (2014) Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147(4): 765-783.
- Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, et al. (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59(3): 898-910.
- Dongiovanni P, Meroni M, Baselli GA, Bassani GA, Rametta R, et al. (2017) Insulin resistance promotes Lysyl Oxidase Like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clin Sci 131(12): 1301-1315.
- Noureddin M, Rinella ME (2015) Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 19(2): 361-379.
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, et al. (2012) Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 18(8): 1279-1285.
- Verrijken A, Francque S, Mertens I, Prawitt J, Caron S, et al. (2014) Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 59(1): 121-129.
- Thögersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, et al. (1998) High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation 98(21): 2241-2247.
- Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, et al. (2012) Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase-and toll-like receptor 4-dependent and retinol-independent mechanism. Molecular and cellular biology 32(10): 2010-2019.
- Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U et al. (2019) Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham Heart Study. Clinical Gastroenterology and Hepatology 17(6): 1157-1164.
- Sookoian S, Castaño GO, Burgueño AL, Rosselli MS, Gianotti TF, et al. (2010) Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 209(2): 585-591.
- Baumeier C, Schlüter L, Saussenthaler S, Laeger T, Rödiger M, et al. (2017) Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Molecular metabolism 6(10): 1254-1263.
- Wu HT, Ou HY, Hung HC, Su YC, Lu FH, et al. (2016) A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia 59(8): 1732-1742.
- Van Gaal LF, Mertens IL, Christophe E (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444(7121): 875-880.
- Meex RC, Watt MJ (2017) Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nature Reviews Endocrinology 13(9): 509-520.
- Zhu J, Wan X, Wang Y, Zhu K, Li C, et al. (2017) Serum fetuin B level increased in subjects of nonalcoholic fatty liver disease: a case-control study. Endocrine 56(1): 208-211.
- Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, et al. (2018) Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? European journal of gastroenterology & hepatology 30(9): 979-985.
- Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, et al. (2010) A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell metabolism 12(5): 483-495.
- Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52(5): 1836-1846.
- Srikanth S, Deedwania P (2016) Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Current hypertension reports 18(10): 76.
- Lozano I, Van der Werf R, Bietiger W, Seyfritz E, Peronet C, et al. (2016) High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutrition & metabolism 13(1): 15.
- Vanhoutte PM (2009) Endothelial dysfunction: the first step toward coronary arterioslerosis. Circ J 73(4): 595-601.
- Roche HM, Gibney MJ (2000) The impact of postprandial lipemia in accelerating atherothrombosis. Journal of cardiovascular risk 7(5): 317-324.
- Gill RM, Belt P, Wilson L, Bass NM, Ferrell LD, et al. (2011) Centrizonal Arteries and Microvessels in Non-Alcoholic Steatohepatitis. The American journal of surgical pathology 35(9): 1400-1404.
- Ying I, Saposnik G, Vermeulen MJ, Leung A, Ray JG, et al. (2011) Nonalcoholic fatty liver disease and acute ischemic stroke. Epidemiology 22(1): 129-130.
- Long MT, Wang N, Larson MG, Mitchell GF, Palmisano J, et al. (2015) Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arteriosclerosis, thrombosis, and vascular biology 35(5): 1284-1291.
- Madan SA, John F, Pyrsopoulos N, Pitchumoni CS (2015) Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: a meta-analysis. European journal of gastroenterology & hepatology 27(11): 1237-1248.
- Iwakiri Y, Shah V, Rockey DC (2014) Vascular pathobiology in chronic liver disease and cirrhosis–Current status and future directions. Journal of hepatology 61(4): 912-924.
- Maslak E, Gregorius A, Chlopicki S (2015) Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacological Reports 67(4): 689-694.
- Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, et al. (2012) Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Laboratory investigation 92(10): 1428-1439.
- Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, et al. (2012) Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PloS one 7(4).
- Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, et al. (2005) Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 42(2): 473-480.
- Kasumov T, Edmison JM, Dasarathy S, Bennett C, Lopez R, et al. (2011) Plasma levels of asymmetric dimethylarginine in patients with biopsy-proven nonalcoholic fatty liver disease. Metabolism 60(6): 776-781.
- Elsheikh E, Younoszai Z, Otgonsuren M, Hunt S, Raybuck B, et al. (2014) Markers of endothelial dysfunction in patients with non‐alcoholic fatty liver disease and coronary artery disease. Journal of gastroenterology and hepatology 29(7): 1528-1534.
- Chiang CH, Huang PH, Chung FP, Chen ZY, Leu HB, et al. (2012) Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One 7(2).
- Farrell GC, Teoh N, McCuskey R (2008) Hepatic microcirculation in fatty liver disease. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 291(6): 684-692.
- Gkaliagkousi E, Douma S (2009) The pathogenesis of arterial stiffness and its prognostic value in essential hypertension and cardiovascular diseases. Hippokratia 13(2): 70-75.
- Zang S, Ma X, Zhuang Z, Liu J, Bian D, et al. (2016) Increased ratio of neutrophil elastase to α1‐antitrypsin is closely associated with liver inflammation in patients with nonalcoholic steatohepatitis. Clinical and Experimental Pharmacology and Physiology 43(1): 13-21.
- Sunbul M, Agirbasli M, Durmus E, Kivrak T, Akin H, et al. (2014) Arterial stiffness in patients with non-alcoholic fatty liver disease is related to fibrosis stage and epicardial adipose tissue thickness. Atherosclerosis 237(2): 490-493.
- Ashino T, Yamamoto M, Numazawa S (2016) Nrf2/Keap1 system regulates vascular smooth muscle cell apoptosis for vascular homeostasis: role in neointimal formation after vascular injury. Scientific reports 6(1): 1-12.
- Huang R, Duan X1, Fan J1, Li G1, Wang B (2019) Role of Noncoding RNA in Development of Nonalcoholic Fatty Liver Disease. BioMed research international 2019.
- Becker PP, et al. (2015) Performance of serum microRNAs-122, -192 and-21 as biomarkers in patients with non-alcoholic steatohepatitis. PloS one 10(11).
- Tan Y, Ge G, Pan T, Wen D, Gan J (2014) A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PloS one 9(8).
- Braunersreuther V, Viviani GL, Mach F, Montecucco F (2012) Role of cytokines and chemokines in non-alcoholic fatty liver disease. World journal of gastroenterology 18(8): 727-735.
- Park SY, Ji‐Soo Lee, Hyung‐Hee Baek, Hyeon Gyu Lee, (2010) Purification and characterization of antioxidant peptides from soy protein hydrolysate. Journal of food biochemistry 34(1): 120-132.
- Miller AM, Gilchrist DS, Nijjar J, Araldi E, Ramirez CM, et al. (2013) MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PloS one 8(8).
- Clarke JD, Sharapova T, Lake AD, Blomme E, Maher J, et al. (2014) Circulating microRNA 122 in the methionine and choline‐deficient mouse model of non‐alcoholic steatohepatitis. Journal of Applied Toxicology 34(6): 726-732.
- Yamada H, Ohashi K, Suzuki K, Munetsuna E, Ando Y, et al. (2015) Longitudinal study of circulating miR-122 in a rat model of non-alcoholic fatty liver disease. Clinica Chimica Acta 446: 267-271.
- Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, et al. (2015) Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut 64(5): 800-812.
- Gao X, Dai M, Li Q, Wang Z, Lu Y, et al. (2017) HMGA 2 regulates lung cancer proliferation and metastasis. Thoracic cancer 8(5): 501-510.
- Guo XY, Sun F, Chen JN, Wang YQ, Pan Q, et al. (2018) circRNA_0046366 inhibits hepatocellular steatosis by normalization of PPAR signaling. World journal of gastroenterology 24(3): 323-337.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...