
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
May Gastrointestinal and Sensory Manifestations be related to Worse COVID-19 Phenotypes Volume 3 - Issue 2
Raquel Susana Torrinhas1, Paulo César Ribeiro2, Yassmin Syagha3, Anna Carolina Pompermayer Coradelli2 and Dan Linetzky Waitzberg1*
- 1Department of Gastroenterology, Laboratory of Nutrition and Metabolic Surgery of the Digestive Tract, University of São Paulo, School of Medicine, Brazil
- 2Charitable Society of Ladies Sírio-Libanês Hospital of São Paulo, Brazil
- 3 Saint Vincent de Paul Charity Hospital, São Paulo, Brazil
Received:February 10, 2022 Published: February 15, 2022
*Corresponding author: Dan Linetzky Department of Gastroenterology, Laboratory of Nutrition and Metabolic Surgery of the Digestive Tract (LIM 35), University of São Paulo, School of Medicine (FMUSP), São Paulo, Brazil
DOI: 10.32474/CTGH.2022.03.000164
Abstract
Extra pulmonary symptoms may contribute to poor outcome in COVID-19. We compared the frequency of gastrointestinal and sensory manifestations (GSM) between mild and severely ill patients with COVID-19, alone or combined with classic respiratory manifestations (CRM). Hospitalized patients with COVID-19 (n = 357) were classified according to their disease severity by using a neutrophile/lymphocyte ratio value ≥18 for severe illness. Presence of CRM and baseline clinical data were recorded. Presence of >3 liquid evacuations per day (diarrhea), decreased usual bowel movements (constipation), nausea/vomiting, lack of appetite (anorexia), abdominal pain, loss of taste (dysgeusia) and/or loss of smell (anosmia) were personally recorded by the investigators at the study admission. Severely ill patients (47.3%) presented worse clinical markers than mildly ill patients, including higher risk of malnutrition and higher need for non-invasive respiratory support (p<0.001). Most of severely ill patients presented at least one GSS (78.1%) and a higher frequency of general and specific GSM (anorexia, constipation, nausea, dysgeusia, and anosmia) than mildly ill patients (p<0.001). The prevalence of CRM without GSM combination was higher in mild illness than in severely ill patients (p<0.001), while the prevalence of GSM without CRM combination was similar between them (p>0.005). Our findings highlight a higher GSM prevalence in severe than in mildly ill patients with COVID-19 presenting CRM, suggesting that GSM may contribute to a worse COVID-19 phenotype when combined to CRM.
Keywords:Anorexia, Diarrhoea; Constipation; Nausea/Vomiting; Abdominal Pain; Dysgeusia; Anosmia; COVID-19
Introduction
Beyond classic respiratory manifestations (CRM), gastrointestinal and sensory (dysgeusia and anosmia) alterations are often described in patients suffering from the coronavirus disease 2019 (COVID-19) [1]. The clinical presentation of COVID‐19 may range from a mild, self‐limiting disease to a multi-organ failure and death [2]. It is unclear whether Gastrointestinal and Sensory Manifestations (GSM) are associated with a more severe disease phenotype either alone or associated to CRM. The neutrophile/ lymphocyte ratio (NLR) have been suggested as a suitable parameter for early-stage prediction of critical illness. Indeed, in COVID-19 patients the NLR proved to be an objective basis for early identification and management of severe pneumonia [3-6]. By using NLR as a disease severity marker, here we characterized the clinical profile of mild and severe illness of COVID-19 hospitalized patients and compared the frequency of GSM between them.
Material and Methods
Study Design and Ethical Issues
This study is part of a larger prospective, multicenter, longitudinal clinical study that enrolled patients from two hospitals in São Paulo (Brazil), Hospital de Caridade São Vicente de Paulo (public) and Hospital Sírio-Libanês (private), and which protocol was approved by the local ethics committee (CAPPesq, nº 4.171.967), registered at the Brazilian Clinical Trials (https://ensaiosclinicos.gov.br/rg/RBR-3h5fr2s) and applied after patients or their legal representatives (when the patient was unable) provided written informed consent.
Patient Selection
A convenience sample of 357 patients was recruited from the wards attended by the Multidisciplinary Nutritional Support Teams at both hospitals. Adult patients (18–90 years old), both genders, any ethnicity, with COVID-19 suspected (lung imaging by computed tomography) or diagnosed (positive polymerase chain reaction) were included. Exclusion criteria were pregnancy, critically ill patients at the Intensive Care Unit (ICU), patients receiving palliative care, and absence of COVID-19 diagnosis confirmed by a positive polymerase chain reaction until the end of their hospital follow-up.
Patient’s stratification according to the disease severity
Patients were stratified according to a disease severity grading, based on the absolute number of neutrophils divided by the absolute number of lymphocytes (NLR). Patients with NLR<18 were considered moderately ill, and those with NLR ≥ 18 were considered severely ill [7].
Descriptive and Clinical Data
Age, sex, ethnicity, body mass index (BMI–usual weight/ height 2), underlying diseases, need for non-invasive respiratory support, presence of CRM (cough and dyspnea), sore throat, clinical complications associated with COVID-19, and administered medications were recorded at the study admission. Up to 36 hours from admission, the nutritional risk screening 2002 tool (NRS-2002) was also applied, where patients with scores ≥ 3 were considered at nutritional risk [8,9]. Hospital discharge was recorded at the study end, where patients non-achieving hospital discharge included those who were admitted to ICU, considered for palliative care, or died.
Prevalence of GSM
Presence of > 3 liquid evacuations per day (diarrhea), decreased usual bowel movements (constipation), nausea/vomiting, lack of appetite (anorexia), abdominal pain, loss of taste (dysgeusia) and/or loss of smell (anosmia) were personally recorded by the investigators at the study admission. Prevalence of GSM was further characterized according to the following categories: absent, at least one, more than one, and combined with respiratory symptoms and/or sore throat.
Statistical Analysis
Continuous variables were expressed using descriptive analysis to measure central tendency (mean or median) or measures of dispersion (standard deviation or minimum-maximum). Categorical variables were expressed as means of percentage values in absolute and relative frequencies. The T-test was performed to compare two continuous variables with a normal distribution (Anderson–Darling test), while the non-parametric Mann–Whitney and Brunner–Munzel tests were used for homogeneous and heterogeneous variables (Bartlett test), respectively. Fisher’s exact test was used for comparisons using categorical variables. Twotailed hypotheses were evaluated, and the confidence intervals were 95%. All statistical analyses were performed using Software R (version 4.0.2) and considering a significance level of 5%.
Results
Patient’s description
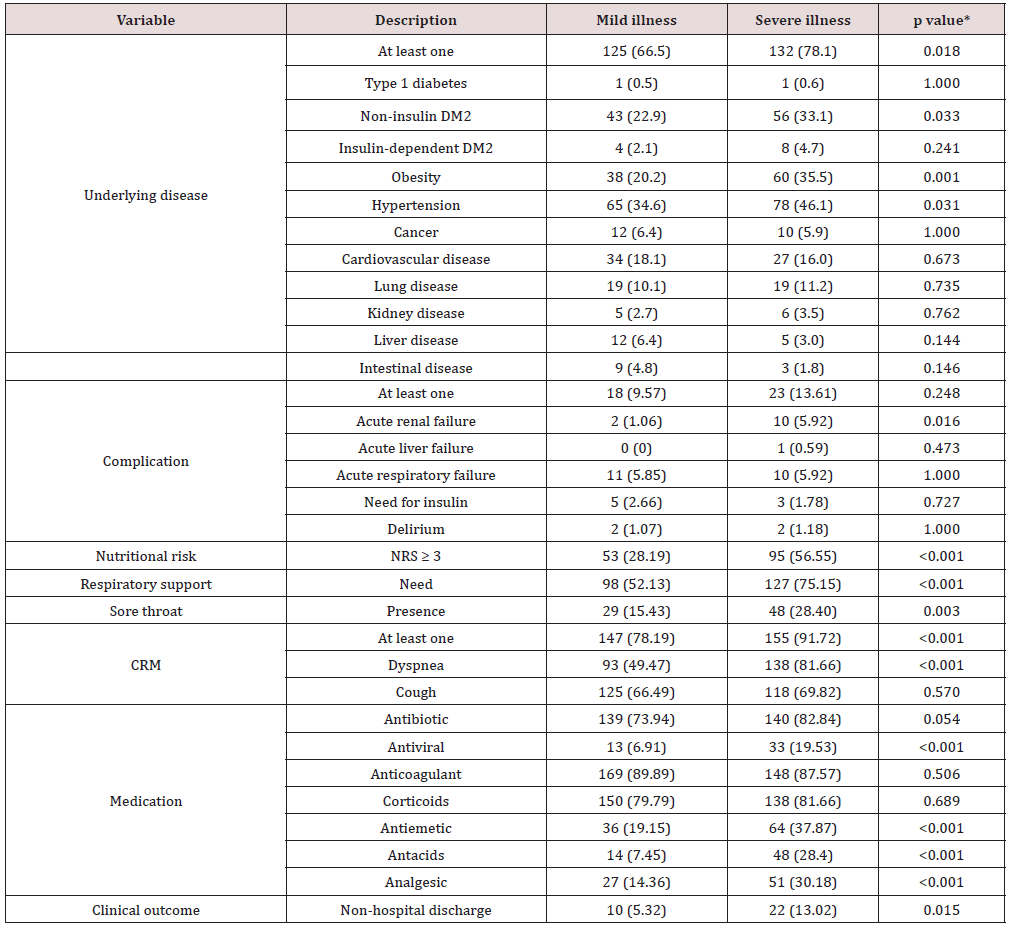
From the 357 patients studied, the majority was male (57.1%), white (54.6%), with a median age of 60 [18–99] years old and a median BMI of 28.3 [15.0–58.4] kg/m2. Almost half of them were severely ill (47.3%), presenting several worse clinical markers than mild illness patients, including higher risk of malnutrition, higher need for non-invasive respiratory support, higher frequency of at least one respiratory manifestation, dyspnea and sore throat, higher frequency of general and specific underlying diseases (previous comorbidities), higher frequency of acute kidney disease as COVID-19 complication, higher requirement for antiviral, antiemetic, antiacid and analgesic medications, and higher frequency of non-hospital discharge (Table 1).
Table 1: Descriptive and clinical characteristics of patients with COVID-19, according to the severity phenotype of the disease.
Data expressed as number of patients (percentage of patients). *Mild illness vs. severe illness. CRM: classic respiratory manifestations; NRS: nutritional risk screening 2002 tool
Prevalence of GSM
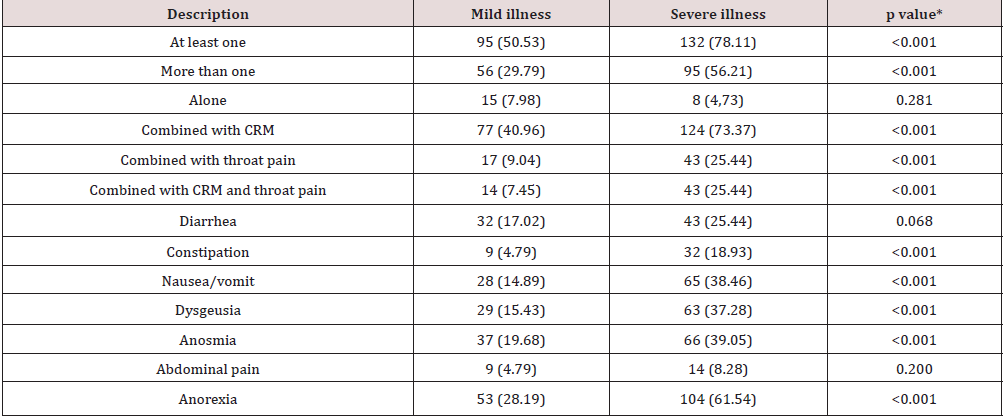
Most patients with severely ill phenotypes presented at least one GSS (78.1%) and a higher GSS frequency than mild illness patients, whether isolated (>1 GSS) or combined to respiratory manifestations or/and sore throat. They also experienced a higher frequency of specific GSS (anorexia, constipation, nausea, dysgeusia, and Anosmia) than mild-illness patients (Table 2). The prevalence of GSM without CRM combination was similar between mild and severe illness patients. On the other hand, the absence of GSM (49.5% vs. 21.9%) and prevalence of CRM without GSM combination (33.5% vs. 15.4%) were higher in mild illness than in severe illness patients (p<0.001).
Table 2: Frequency of gastrointestinal and sensory symptoms in patients with COVID-19, according to the severity phenotype of the disease.
Data expressed as number of patients (percentage of patients). *Mild illness vs. severe illness. CRM: classic respiratory manifestations.
Discussion
Extra pulmonary symptoms in COVID-19 may also contribute to poor outcome. By stratifying COVID-19 patients according to the disease severity we found 78.1% of our severe illness patients with at least one GSM, exhibiting a higher frequency of GSM combined to respiratory signal/symptoms than mild illness patients. On the other hand, the absence of GSM or respiratory manifestations alone was significantly more frequent in mild than in severe illness patients, while the frequency of GSM alone was similar between them. Our findings suggest that GSM may contribute to worsen COVID-19 phenotypes.
Accordingly, Han et al. observed that COVID-19 patients with mild disease presenting digestive symptoms are more likely to test positive for viral RNA in stool, have a longer delay before viral clearance, and experience delayed diagnosis than those presenting only respiratory symptoms [10]. In a meta-analysis, COVID-19 patients with gastrointestinal involvement tended to have a poorer disease course (e.g., acute respiratory distress syndrome) [11]. Another meta-analysis highlighted a higher frequency of gastrointestinal symptoms in severe disease [12].
Our data support that GSM may be related to worse COVID-19 phenotypes, but they also suggest that severe disease may primarily occur when GSM are associated with CRM. Nevertheless, most COVID-19 patients with initial gastrointestinal complaints seems to develop CRM within few days [13]. For instance, in a sample of 206 afflicted patients, the frequency of digestive symptoms alone and combined with CRM was 23,3% and 33.5%, respectively [10]. In our sample of 357 afflicted patients, only 7.3% of them presented GSS not combined with CRM.
In our study, anorexia was the most prevalent GSM (44%) and also was more frequent among severe illness (61.5%) than in mild illness (28.2%) patients. Lack of appetite can impair food intake and further contribute to body weight loss and malnutrition. Severely ill patients with COVID-19 may suffer additional nutritional burdens, due to catabolism and increased energy requirements to fight severe infection [14]. It is possible that GSM may contribute to more severe phenotypes of COVID-19 by impairing energy-protein intake.
In our study, stratification of COVID-19 patients was done using NLR as a disease severity marker. The cut-off value for NLR here applied was based on a COVID-19 patients’ study that categorized NLR values into quartiles (4.61, 11.3, 19.3, and ≥19.3), where the highest NLR quartile was significantly associated with older age and mortality [7]. This NLR classification distinguished the severely ill patients with worse clinical patterns and lower hospital discharge from those with mild illness. We, by using NLR ratio, could identify worse COVID-19 phenotypes, and suggest the adopted cutoff as an efficient parameter for this purpose.
Conclusion
In summary, our findings highlight a higher GSM prevalence in severe than in mild illness patients with COVID-19, suggesting that these extra pulmonary manifestations may contribute to worse COVID-19 phenotypes when combined to CRM. Anorexia was prevalent suggesting that nutritional supplementation may be a relevant intervention to prevent poor clinical outcomes in COVID-19 patients manifesting GSM. Finally, NLR with a cutoff value of ≥ 18 showed a good performance to identify worse clinical phenotypes and could be considered to identify more vulnerable COVID-19 patients.
Acknowledgements
We thank Bianca Zanchetta Buani Miguel, Thaís Nunes Freire, Marli Alves Ramelho da Silva, Fabiana Ruotolo, Daniela Hummel de Almeida, Janayna Nayara Buzato, and Henrique Oliveira e Silva for the kindly support in collecting the study data.
References
- Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, et al. (2020) AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 159(1): 320-334.e27.
- Song JW, Zhang C, Fan X, Meng FP, Xu Z, et al. (2020) Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun 11(1): 3410.
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, et al. (2020) Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 18(1): 206.
- Nalbant A, Kaya T, Varim C, Yaylaci S, Tamer A, et al. (2020) Can the neutrophil/lymphocyte ratio (NLR) have a role in the diagnosis of coronavirus 2019 disease (COVID-19)? Rev Assoc Med Bras (1992) 66(6): 746-751.
- Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, et al. (2020) Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int J Clin Pract 75(4): e13698.
- Yılmaz E, Ak R, Doğanay F (2022) Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of COVID-19 patients: a retrospective cohort study. Sao Paulo Med J 140(1): 81-86.
- Jimeno S, Ventura PS, Castellano JM, García-Adasme SI, Miranda M, et al. (2021) Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur J Clin Invest 51(1): e13404.
- Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, et al. (2020) ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr Edinb Scotl 39(6): 1631–1638.
- Kondrup J, Allison SP, Elia M, Vellas B, Plauth M (2003) Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr 22(4): 415–421.
- Han C, Duan C, Zhang S, Spiegel B, Shi H, et al. (2020) Digestive Symptoms in COVID-19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol 115(6): 916–923.
- Mao R, Qiu Y, He JS, Tan JY, Li XH, et al. (2020) Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 5(7): 667–678.
- Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, et al. (2020) Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 159(1): 81–95.
- Bilal M, Sawhney MS, Feuerstein JD (2021) Coronavirus disease-2019: implications for the gastroenterologist. Curr Opin Gastroenterol 37(1): 23-29.
- Wischmeyer PE (2018) Nutrition therapy in sepsis. Crit Care Clin 34(1): 107-125.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


