Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
Is there a utility of Nordin’s Index in the evaluation of osteoporosis in patients with post viral cirrhosis? Results of a pilot study in Cameroonians Volume 3 - Issue 1
Mathurin Pierre Kowo1,2*, Raoul Rigobert Dim Bassi1, Jan René Nkeck1, Antonin Wilson Ndjitoyap Ndam3, Vicky Jocelyne Ama Moor1,2, Firmin Ankouane Andoulo1,4, Isabelle Dang Babagna Timba3 and Madeleine Singwé Ngandeu1,4
- 1Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
- 2University Hospital Centre, Yaoundé, Cameroon
- 3Yaoundé General Hospital, Yaoundé, Cameroon
- 4Yaoundé Central Hospital, Yaoundé, Cameroon
Received: November 11, 2020; Published: December 17, 2020
*Corresponding author: Mathurin Pierre Kowo,Faculty of Medicine and Biomedical Sciences, University Hospital Centre,University of Yaoundé I, Cameroon
DOI: 10.32474/CTGH.2020.03.000155
Abstract
Background: Metabolic bone disorders are frequent in patients with cirrhosis. These two conditions are usually misdiagnosed in Sub-Saharan Africa before the onset of complications. A full evaluation of metabolic bone disorders in patients with liver cirrhosis is difficult in our milieu and osteodensitometry is rarely available. In this preliminary study, we sought to determine the necessity of a cost-effective method, the Nordin’s Index, in the evaluation of osteoporosis in patients with liver cirrhosis.
Methods: This was a prospective cross-sectional study from January to May 2017 in Yaoundé. We compared the data of 19 patients (15 men, 4 women) with post viral liver cirrhosis and 17 controls (13 men, 4 women) paired with age, gender and body mass index (BMI). Data collected included vitamin D levels, serum and urine concentrations of calcium and phosphorus, Nordin’s Index and results of the bone mineral density using an x-ray absorptiometry scan. Statistical analysis was performed using the software SPSS 21. A p value of less than 0.05 was considered to be statistically significant.
Results: The mean age of patients was 38 ±15 years, with a mean BMI of 25 ±8 kg/m². Three of the four women were on menopause. Etiologies of cirrhosis were viral hepatitis B (8 patients), viral hepatitis B and D coinfection (7 patients), and viral hepatitis C (4 patients). The median duration of cirrhosis was 19 [8; 48] months, and 14 patients were classified grade A in Child Pugh classification. There was no statistical difference in the serum and urine concentrations of calcium and phosphorus. Osteoporosis was more frequent in cirrhosis (31.6% versus 11.8%, p<0.05). Nordin’s Index was significantly elevated in patients with cirrhosis compared to controls (0.12 [0.06; 0.13] mg/mg, versus 0.03 [0.01; 0.08] mg/mg, p<0.05), and in patients with cirrhosis associated to osteoporosis compared to those without (0.13 [0.09; 0.13] mg/mg versus 0.07 [0.03; 0.08] mg/mg, p<0.05). Vitamin D deficiency was more observed in controls (13/17 versus 7/19, p<0.05). Factors associated with osteoporosis were disease duration, elevated Nordin’s Index and elevated serum level of transaminases.
Conclusion: The Nordin’s Index, a simple and inexpensive tool for exploration of the phosphocalcic metabolism, could be useful for the evaluation of osteoporosis during viral cirrhosis. However, its performance has to be evaluated in a larger sample.
Keywords: Cirrhosis, chronic viral hepatitis, osteoporosis, Nordin’s Index, Vitamin D.
Abbreviations: ALAT: Alanine Aminotransferase; ALP: Alkaline Phosphate; ASAT: Aspartate Aminotransferase, BMD: Body Mass Density; GGT: Gamma Gluthamyl Transferase
Introduction
Cirrhosis is the outcome of most chronic liver diseases. It
remains a major public health problem in Sub-Saharan Africa
and particularly in Cameroon, where chronic viral hepatitis is
highly endemic [1-3]. In fact, sixty percent of cirrhosis in Africa
are attributable to viral hepatitis B and C [4]. In Cameroon,
70.9% of cirrhosis cases are due to chronic viral hepatitis B and
25.5% to chronic viral hepatitis C [5]. The hallmark of cirrhosis is
hepatocellular insufficiency and portal hypertension, resulting in
impaired liver function and subsequent systemic abnormalities.
Bone damage is one of the systemic abnormalities frequently
seen in cirrhosis, regardless of etiology, and is known as hepatic
osteodystrophy [6].
Hepatic osteodystrophy is a combination of osteoporosis and
osteomalacia, the latter being rare during cirrhosis [6]. According
to the World Health Organization (WHO), osteoporosis is a diffuse
disease of the skeleton, characterized by a decrease in bone mass
and an alteration of the micro-architecture of bone tissue, leading
to increased bone fragility and an increased risk of fractures [7]. Its
pathogenesis during cirrhosis is complex and leads to an increase in
bone resorption by osteoclasts to the detriment of its formation by
osteoblasts [8]. The prevalence of osteoporosis during cirrhosis is
between 12% and 55% [9]. In Africa, more precisely in the Maghreb
region, it has been estimated to 28.26% [10]. Osteoporosis is
almost asymptomatic until complications. It is a real public health
problem as it causes low energy fractures in more than 40% of
cases, affecting the morbidity and quality of life of patients with
cirrhosis [8]. Several factors are associated with the occurrence of
osteoporosis during cirrhosis, including vitamin D deficiency with
a prevalence of 32% in Europe and 32.6% in Africa during cirrhosis
[10,11]; Elevated Child and Pugh Score, duration of disease, etiology
of cirrhosis, low body mass index, with some controversy in the
literature [12].
Considering the risk of osteoporosis being high during cirrhosis,
it is necessary to have an evaluation of bone mineral density in
these patients [13,14]. However, this is rare in our context because
of the cost and accessibility of this examination. With the absence of
hepatic transplantation in our environment to cure cirrhosis, there
is a long duration of cirrhosis and, therefore, an increased risk of
complications including osteoporosis. It is therefore important to
use simple and inexpensive tools to make the diagnosis or at least
to detect high-risk subjects. Nordin’s Index is calculated by the
calciuria/creatinuria ratio. It is an old marker of bone resorption,
which correlates with post-menopausal osteoporosis and its risk
of fracture [15,16]. However, its usefulness in cirrhosis especially
post viral has been little studied to the best of our knowledge. In
this study, we sought to determine the utility of Nordin’s Index as
a cost-effective method in the evaluation of osteoporosis in people
suffering from post-viral cirrhosis in a highly endemic area for
chronic viral hepatitis and limited access to bone mineral density
assessment.
Patients and Methods
Study design
The study used a cross sectional design with a prospective data collection.
Study setting
This study took place from January to May 2017 at the Yaoundé Central Hospital and Cathedral Medical Centre (CMC) at Yaoundé, where the participants were recruited. Laboratory analysis were conducted at the University Hospital Centre of Yaoundé. Bone mineral density measurements were carried out at the Autonomous Centre for Radiology and Medical Imaging (CARIM) at Yaoundé.
Participants
The sample was made up of 19 adult volunteers (15 men and
4 women) followed for a cirrhosis of viral cause (chronic viral
hepatitis B, C, and D). They were matched with 17 adult volunteers
(13 men and 4 women) without any chronic or acute disease/
condition, paired with age (±2 years), gender and body mass index
(BMI; ±2 kg/m²). The diagnosis of liver cirrhosis was based on
clinical and biological signs of portal hypertension and chronic
liver failure, ultrasonographic signs of chronic liver disease and
endoscopic signs of portal hypertension. Ultrasonographic signs
included irregular liver outline, heterogeneous echo structure,
dysmorphic liver, enlarged portal vein and presence of collateral
venous circulations.
The controls were recruited from among the patients’
caregivers to ensure equal exposure to environmental and
nutritional factors that may contribute to osteoporosis. We did not
include any participants who presented pathologies with known
repercussions on bone and/or phosphocalcic metabolism, such as:
chronic renal disease, thyroid and parathyroid disorders, Cushing
Syndrome, diabetes, HIV infection and cancer. We did not include
participants receiving treatment with known effects on bone and/
or calcium phosphorus metabolism such as: hormone replacement
therapy, biphosphonates, calcium, vitamin D, corticosteroids,
antimetabolites, anticoagulants, anticonvulsants, thyroxine.
Pregnant women instead of women with amenorrhea, participants
with diabetes, tobacco and alcohol consumers were not included.
Sample size calculation
The sample size was estimated using the formula in Whitley et al. to compare proportions between groups [17]. The parameters used for the calculation of the standardized difference were derived from the study of Goral et al [18]. They found a mean T-score of -1.6 SD in patients with cirrhosis and -0.25 SD in controls, with a standard deviation of 1.3 SD. In our study, the level of significance was set at p 0.05 and the power at 80%. The minimum sample per group was 15.
Procedure
Ethical considerations
We obtained research authorizations in the selected health care facilities and the approval of the Institutional Review Board of the Faculty of Medicine and Biomedical Sciences. All the participants read and signed an informed consent form.
Clinical data
Sociodemographic (age, gender) and clinical data were collected through a questionnaire. Clinical variables were anthropometric parameters (weight and height) for BMI calculation, the history of cirrhosis (duration, etiology, complications, current Child and Pugh’s Score, treatment), suggestive signs of osteoporosis and its complications (history of pathological and or low energy fracture), comorbidities, menopause for women and its duration, and family history of osteoporosis.
Laboratory Analysis
For laboratory analysis, 10 mL of peripheral venous blood sample was collected from each participant after eight hours of fasting. The blood samples were centrifuged for 5 minutes at 3000 rpm using the Humax 14K Germany centrifuge. 500μl of serum was then extracted for the determination of calcium (mg/L), phosphorus (mg/L), albumin (g/L) by colorimetric method and for the determination of creatinine (mg/l), total alkaline phosphatase (U/L), transaminase (Aspartate amino transferase (ASAT), Alanine amino transferase (ALAT), in U/L), gamma gluthamyl transferase (GGT, U/L) by kinetic method, using the Mindray model BS 120 spectrophotometer. The remaining serum was stored at -20° Celsius and used to determine 25(OH) vitamin D (ng/mL) levels by ELISA using the BioTek EL×800 ELISA chain. Serum creatinine was used to estimate the glomerular filtration rate using four parameters’ MDRD formula in mL/min for 1.72m². The measured calcemia was corrected using albuminemia. Hypercalcemia was defined for a corrected Calcemia > 104 mg/L, while Hypocalcemia was defined below 81 mg/L [19]. Hyperphosphatemia was defined as blood phosphorus > 50 mg/L in adults while hypophosphatemia was defined below 25 mg/L [19]. Vitamin D Status was assessed according to the classification of Holick et al [20].
Laboratory Analysis
After consumption of 200 ml of low calcium water (Volvic® type: Ca<10 mg) in a fasting individual with a previously empty bladder, calciuria and creatininuria was measured on the urine collected 2 hours later. Nordin’s Index was calculated: calciuria/ creatininuria (mg/mg). The normal value of the Nordin’s Index is lower than 0.11 mg/mg. An increase in the index indicates hyperresorption of bone. Calcium, phosphorus and creatinine in urine were determined using the same methods as in the plasma.
Bone Mineral Density (BMD)
Bone mineral density was measured by biphotonic X-ray absorptiometry scan using the Hitachi® lunar prodigy DXA system, with 37 Micro SieVert (μSV) dose per measurement site. Bone mineral density was measured at the lumbar spine, the neck of the left femur and the distal end of the left radius. Data on bone mineral density were expressed in g/cm², T-score and Z-score. Osteoporosis was defined as T-score <-2.5 for patients aged 50 years and older or a Z-score <-2 for patients under 50 years of age for measurement of the lumbar spine, femoral neck or distal end of the radius [21].
Statistical analysis
Statistical analysis was performed using SPSS version 21.0. Quantitative variables were expressed as mean ± standard deviation or median and interquartile range [25th and 75th quartiles]. Qualitative variables were expressed as effectives and proportions. The Mann-Whitney test was used to compare the means, while medians were compare using the Median’s test. Fisher Exact Test was used to compare proportions. We searched for associations between continuous variables and bone mineral density on various sites by calculating the Pearson’s Correlation Coefficient to determine the factors associated with the decrease in BMD. For all the tests, the statistical significance level was set at 0.05.
Results
Clinical characteristics of the study samples
The mean age of patients was 38.6 ±15.9 years, while that of controls was 37.8 ±15.9 years (p>0.05). Participants’ ages ranged from 23 to 73 years. There were respectively 2/4 and 2/4 women on menopause in the patients and control groups. Between cases and controls, there was no statistical difference in body mass index (p>0.05) (Table 1). The median duration of cirrhosis was 19 months, range from one to 120 months. The causes of liver cirrhosis were chronic viral hepatitis B (42%), hepatitis C (31%), and B-delta (37%). There was no coinfection with viral hepatitis B and C. Most of the cases were classified on stage A (n=14), while 3 and 2 cases were respectively on-stage B and C in Child and Pugh classification.
Bone and calcium-phosphate metabolism
Between patients and controls, there was no difference in serum levels of ASAT, ALAT, GGT. Albuminemia was significantly lower in cirrhotic patients (33.7 ±8.6 g/L versus 42.6 ±2.5g/L, p<0.001). Serum total alkaline phosphatase levels were significantly higher in patients with cirrhosis (141.4 ±60.9 U/L versus 72.2 ±28.9U/L, p<0.001). There was no difference in serum and urinary excretion of calcium and phosphorus. Vitamin D levels were higher in patients with cirrhosis (22.8 ±7.1ng/mL versus 11.3 ±5ng/mL, p<0.05), and the frequency of vitamin D deficiency was significantly high in controls (14 (82.3) versus 7 (36.8), p<0.05). The median of Nordin’s Index was significantly higher in cases compared to controls (0.12 [0.06; 0.13] mg/mg versus 0.03 [0.01; 0.08] mg/mg, p<0.05) and in patients with cirrhosis who have osteoporosis compared to those without (0.13 [0.09; 0.13] mg/mg versus 0.07 [0.03; 0.08] mg/mg, p<0.05) (Table 1).
Table 1. Clinical and biological characteristics of the study samples.
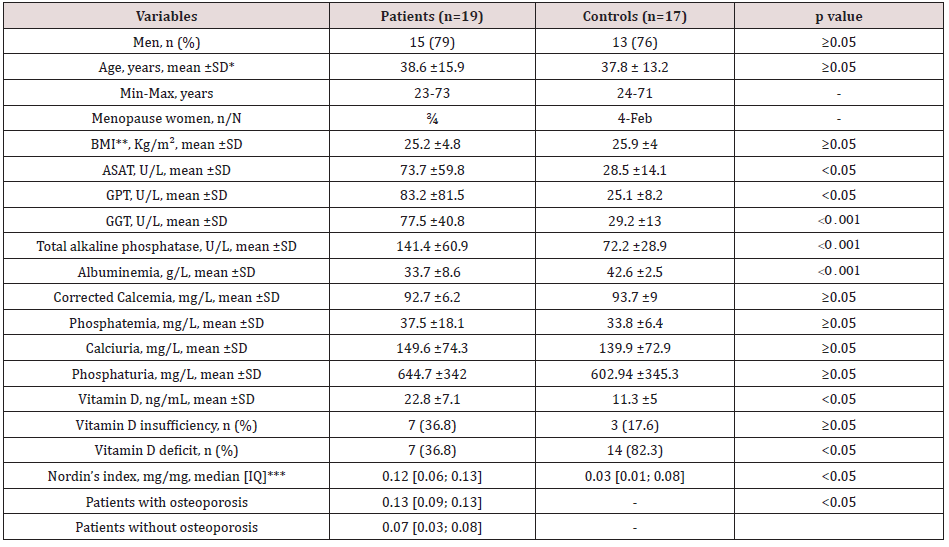
SD: Standard deviation; **BMI: Body Mass Index; ***IQ: interquartile range
Osteoporosis and associated factors
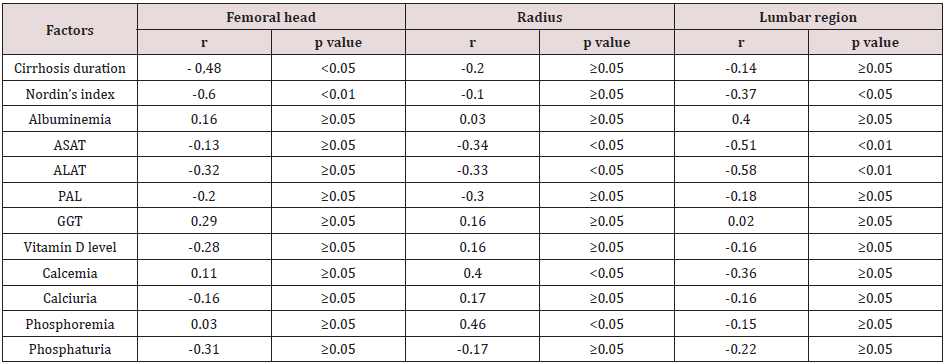
The frequency of osteoporosis was three times higher in the test group (31.5%) than in the control group (11.7%) (p<0.05). The most frequent site of osteoporosis was radius (6/19 for cases and 2/17 for controls). There was only one case of osteoporosis on the femoral neck in controls, and one case on the lumbar region in cases. Several biomarkers were found to be associated with the decrease in BMD at the different sites. These included the duration of cirrhosis, which was weakly and inversely correlated with decreased BMD at the femoral head and distal end of the radius. We found a weak and moderately negative correlation between the Nordin’s Index and the BMD at the femoral and lumbar levels respectively. There were also weak correlations between transaminases levels (ASAT, ALAT), corrected calcemia and phosphoremia, with BMD values at the radial and lumbar levels. These associations are presented in (Table 2).
Discussion
Osteoporosis is by far the most frequent bone complication
during cirrhosis irrespective of the etiology. Thus, it remains
a concern for the specialist. Osteodensitometry is the exam of
choice in the investigation of osteoporosis in cirrhotic patients.
However, due to its high cost and low availability, it is not commonly
prescribed. There is also no subsidies for paraclinical examinations
of patients affected by cirrhosis in our context, which is highly
endemic with chronic viral hepatitis. We conducted this study as
a pilot study with the aim of evaluating markers and indices that
are common, accessible and easy to perform in order to investigate
bone metabolism abnormalities in post viral cirrhosis. Our results
show that in addition to transaminase abnormalities, whose high
levels are associated with a decrease in BMD, the Nordin’s Index
could be an evaluation tool, as it is better correlated with a decrease
in BMD.
Being the main constituents of bone, we first explored markers
of phosphocalcic metabolism. We found a similar plasma and urine
calcium-phosphorus profile between the cirrhotic patients and
the controls. As previously reported in the literature, despite the
alteration in BMD, there is an increase in parathyroid hormone
that is not related to secondary hyperparathyroidism, but rather
to the alteration of liver function [22]. Thus, ensuring calciumphosphorus
balance. This explanation was confirmed by Fisher et
al., who found that despite vitamin D deficiency in patients with
cirrhosis, their calcium-phosphorus metabolism was balanced,
and they did not present secondary hyperparathyroidism [23].
The level of 25(OH) vitamin D which is a reflection of vitamin D
storage, was significantly increased in patients with cirrhosis.
25(OH) vitamin D is synthesized from 7-dehydrocholesterol under
the action of 25 hepatic hydroxylase. Hepatic failure observed
during cirrhosis leads to a defect in vitamin D hydroxylation and a
defect in the production of albumin and vitamin D binding proteins
(VDP) with a consequent drop in vitamin D levels [24,25]. The
higher incidence of vitamin D deficiency in the control group may
be a reflection of their diet which was not assessed in our study.
More so, there are several other exogenous factors associated with
vitamin D deficiency namely: lack of sunlight (main cause), lack of
oral vitamin D intake, and black skin [26, 27,28].
We assessed bone formation by evaluating serum total
alkaline phosphatase levels. The significant elevation of alkaline
phosphatases, coupled with gamma GT would, however, be more
a reflection of cholestasis accompanying liver damage rather than
a disturbance on bone metabolism. We also observed a weak and
negative correlation between transaminase levels and BMD in
people with cirrhosis in some sites. This result is similar to that
found by Turkeli et al. and Karan et al [29,30]. However, since
these markers are not markers of bone and calcium-phosphorus
metabolism, this correlation would be more reflective of the
cytolysis induced by viral hepatitis.
Evaluation of bone status requires biological assays of
bone remodeling and measurement of bone mineral density by
biphotonic X-ray absorptiometry. However, the bone resorption
could be evaluated with the Nordin’s Index. This marker was
elevated in 63.2% of patients with cirrhosis compared to 11.8%
in the control group, with a significant increase in patients with
cirrhosis. This result is similar to those found in the literature
suggesting increased bone resorption in patients with cirrhosis.
Bone mineral density assessment by biphoton X-ray absorptiometry
found a greater frequency in osteoporosis in cirrhotic patients,
and correlates literature findings which reported a prevalence
between 15 and 55% [9]. The high risk of having osteoporosis
during cirrhosis is explained by an increase in bone resorption in
these patients as found in our study and described in the literature
[8,24]. The most affected site in our study was the distal end of the
radius followed by the lumbar spine and femoral neck, respectively;
similar to the findings of Diamond et al [31]. However, few authors
assessed bone mass at the distal end of the radius in their study.
The predominance of osteoporosis at the distal end of the radius
and lumbar spine is explained by the fact that they consist of 60%
and 50% trabecular bone, respectively, compared to the femoral
neck, which is 60% cortical bone [32]. Indeed, trabecular bone
has an accelerated metabolism and early demineralization during
pathologies with repercussions on bone metabolism as opposed
to bone that has a slower metabolism and slow demineralization.
In association with decrease in BMD, disease duration, an elevated
Nordin’s Index were negatively correlated. However, vitamin D
levels, transaminases levels and the other markers were either
weakly or not at all correlated.
The evaluation of the Nordin’s Index may be carried out
during post viral cirrhosis in low-income countries. However, the
interpretation of the results of our study must be done with certain
reservations, notably the small size of our sample and the absence of multiple assays knowing that the concentrations of the biomarkers
evaluated could vary over time. A study on a larger sample would
thus be necessary to better specify these results.
Conclusion
The estimation of the Nordin’s Index could be helpful for the evaluation of osteoporosis in patients with chronic liver disease. However, its performance has to be evaluated in a large sample.
Declaration
Ethical approval and consent to participate. Research authorizations were obtained from the administration of the University Hospital Centre of Yaoundé. An ethical clearance was obtained from the Institutional Ethical Review Board of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I (Cameroon). All the patients read and signed an informed consent sheet.
Consent for publication
Not applicable.
Availabilityof data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interest
The authors declared there is no conflict of interest.
Authors contribution
MPK and MSN designed the study; RRDB, IDBT collected data. RRDB and VJAM analyzed data. JRN, RRDM and MPK built the manuscript; AWNN, FAA and VJAM revised the manuscript; All the study was done under the supervision of MSN. All authors read and approved the final manuscript.
Acknowledgement
The authors would like to thank Dr. Godfrey Esoh for his substantial contribution to improve the language of this manuscript.
References
- Bigna JJ, Amougou MA, Asangbeh SL, Kenne AM, Noumegni SRN, et al. (2017) Seroprevalence of hepatitis B virus infection in Cameroon: A systematic review and meta-analysis. BMJ Open 7(6).
- Kowo MP, Andoulo FA, Ngek LT, Sizimboue DT, Ndam AN, et al. (2019) Prevalence of Hepatitis C Virus and Associated Risk Factors among Inmates at New Bell Prison, Douala, Cameroon. Open J Epidemiol 9(2):119-1
- Bigna JJ, Nkeck JR, Ngouo A, Nyaga UF, Noubiap JJ (2018) Hepatitis B virus and HIV coinfection among adults residing in Cameroon: A systematic review and meta-analysis of prevalence studies. Infect Dis Health23(3): 170-17
- Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, et al. (2020) The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5(3):245-2
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepato 45(4): 529-538.
- Idilman R, de Maria N, Uzunalimoglu O, van Thiel DH (1997) Hepatic osteodystrophy: A review. HepatogastroenterologyAvr 44(14):574-5
- (1993) Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med94(6): 646-6
- Nakchbandi IA (2014) Osteoporosis and fractures in liver disease: Relevance, pathogenesis and therapeutic implications. World J Gastroenterol WJG20(28): 9427-94
- Gatta A, Verardo A, Di Pascoli M, Giannini S, Bolognesi M (2014) Hepatic osteodystrophy. Clin Cases Miner Bone Metab11(3): 185-1
- Goubraim R, Kabbaj N, Salihoun M, Chaoui Z, Nya M, et al. (2013) Metabolic Bone Disease in Viral Cirrhosis: A Prospective Study. ISRN Hepatol 2013:276563.
- George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, et al. (2009) Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol WJG15(28): 3516-35
- Hajiabbasi A, Shafaghi A, Fayazi HS, Shenavar I (2015) The Factors Affecting Bone Density in Cirrhosis. Hepat Mon15(4): e26871.
- Yadav A, Carey EJ (2013) Osteoporosis in chronic liver disease. Nutr Clin Pract 28(1): 52-
- Santos LAA, Romeiro FG (2016) Diagnosis and Management of Cirrhosis-Related Osteoporosis. BioMed Res Int 1423462.
- S Busi, A Catalano (1995) Reduction of the Nordin index after therapy with oral alendronate in patients with postmenopausal osteoporosis. Clin Ter 146(12): 857-85
- (1996) Cumulated Index Medicus U.S.A. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Library of Medicine. pp.1344.
- Whitley E, Ball J (2002) Statistics review 4: Sample size calculations. Crit Care Lond Engl 6(4): 335-3
- Goral V, Simsek M, Mete N (2010) Hepatic osteodystrophy and liver cirrhosis. World J Gastroenterol WJG 16(13): 1639-16
- Analyses de biologiemédicale: Valeursusuelles [Internet]. [cité 2 déc 2016].
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. (2011) Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96(7): 1911-19
- 2015 ISCD Official Positions - Adult - International Society for Clinical Densitometry (ISCD).
- Kirch W, Höfig M, Ledendecker T, Schmidt-Gayk H (1990) Parathyroid hormone and cirrhosis of the liver. J Clin Endocrinol Metab 71(6): 1561-1566.
- Fisher L, Fisher A (2007) Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol 5(4): 513-520.
- López-Larramona G, Lucendo AJ, González-Castillo S, Tenias JM (2011) Hepatic osteodystrophy: An important matter for consideration in chronic liver disease. World J Hepatol 3(12): 300-30
- Iruzubieta P, TeránÁ, Crespo J, Fábrega E (2014) Vitamin D deficiency in chronic liver disease. World J Hepato 6(12): 901-9
- Allali F, El Aichaoui S, Khazani H, Benyahia B, Saoud B, et al. (2009) High prevalence of hypovitaminosis D in Morocco: Relationship to lifestyle, physical performance, bone markers, and bone mineral density. Seminars in arthritis and rheumatism 38(6): 444-451.
- Holick MF, Chen TC (2008) Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr 87(4): 1080S-1086S.
- Prentice A, Schoenmakers I, Jones KS, Jarjou LMA, Goldberg GR (2009) Vitamin D Deficiency and Its Health Consequences in Africa. Clin Rev Bone Miner Metab mars (1): 94-
- Turkeli M, Dursun H, Albayrak F, Okçu N, Uyanik MH, et al. (2008) Effects of Cirrhosis on Bone Mineral Density and Bone Metabolism. Eurasian J Med avr 40(1): 18-
- Karan MA, Erten N, Tascioglu C, Karan A, Sindel D, et al. (2001) Osteodystrophy in posthepatitic cirrhosis. Yonsei Med J 42(5): 547-552.
- Diamond T, Stiel D, Lunzer M, Wilkinson M, Roche J, et al. (1990) Osteoporosis and skeletal fractures in chronic liver disease. Gut janv 31(1): 82-8
- De Vernejoul Pierre Marie M-C (1993) Cellules osseuses et remodelageosseux. MS Médecine Sci 9(11): 1192-1203.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...