Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Opinion(ISSN: 2641-1652) 
Indigenous and Spiked-Pancreatic Intraepithelial Neoplasia Volume 4 - Issue 2
Anubha Bajaj*
- Consultant Histopathology, Punjab University, India
Received: March 06, 2023; Published: March 28, 2023
*Corresponding author: Anubha Bajaj, Consultant Histopathology, Punjab University, India
DOI: 10.32474/CTGH.2023.04.000183
Opinion
Pancreatic intraepithelial neoplasia (PanIN) configures as a neoplastic lesion of pancreas discernible upon microscopic evaluation. The lesion demonstrates a potential of progressing into invasive ductal adenocarcinoma. Besides, the condition signifies as a predominant precursor lesion leading up to invasive pancreatic adenocarcinoma. Pancreatic intraepithelial neoplasia configures as a miniature, papillary or flattened, non-invasive epithelial neoplasm. Characteristically, the lesion exhibits a spectrum of variable cytological and architectural atypia along with varying proportions of mucin secretion. Occasionally, the condition is discerned incidentally within resected pancreatectomy specimens when the surgical manoeuver is performed for diverse disorders. By definition, pancreatic intraepithelial neoplasia manifests a non-tumoral variety of cellular dysplasia or intraepithelial neoplasia. Thus categorized, the lesion is devoid of cogent clinical manifestations. Generally, lesions are miniature <0.5-centimetre magnitude wherein gross or radiologic assessment may be challenging. Carcinoma in situ is contemplated as a high-grade lesion which warrants meticulous clinical monitoring.
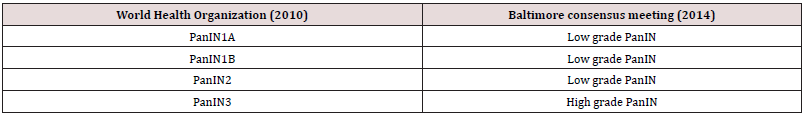
Enhancing age is associated with elevated disease frequency. Low grade lesions emerge as a frequent incidental finding discerned within >50% population exceeding> 50 years of age. High grade lesions are commonly associated with invasive ductal adenocarcinoma. No site of disease emergence within pancreas is exempt. The majority of instances are encountered within pancreatectomy specimens resected for pancreatic ductal adenocarcinoma. In contrast to the main pancreatic duct, pancreatic intraepithelial neoplasia is commonly observed within branch ducts. The metamorphosis may concur within heterotopic pancreas. Preliminary molecular modifications discerned within pancreatic intraepithelial neoplasia emerge as telomere shortening, upregulation of p21 or oncogene activation of KRAS as encountered within low grade pancreatic intraepithelial neoplasia1. Intermediate modifications as inactivation of tumour suppressor gene p16 CDKN2A may arise. Delayed disease stage is associated with inactivation of BRCA2, SMAD4 (DPC4) and TP53 tumour suppressor gene. Pancreatic intraepithelial neoplasia is devoid of cogent clinical manifestations and commonly abuts pancreatic ductal adenocarcinoma [1,2].
Grossly, pancreatic intraepithelial neoplasia remains undetectable. Occasionally, lesions induce ductal obstruction with the consequent occurrence of subtle fibrosis within upstream pancreatic tissue. By definition, pancreatic intraepithelial neoplasia arises as incidental microscopic lesions which are nonvisible upon clinical or radiographic assessment. Therefore, lesions are unamenable to procedural maneuvers as fine needle aspiration. Exceptionally, lesions which appear as a component of aspiration material configure as mucinous glandular epithelium with atypical alterations. However, categorization of pancreatic intraepithelial neoplasia upon cytology may remain non confirmatory. Classically, affirmation of pancreatic intraepithelial neoplasia upon cytological preparations is to be circumvented. Upon microscopy, tumour magnitude appears < 0.5 centimeters. Low grade pancreatic intraepithelial neoplasia 1A manifests as a preliminary precursor lesion constituted of flattened columnar epithelium or tall, columnar, mucin secreting cells with miniature, spherical to elliptical, basal nuclei. Neoplastic cells are essentially devoid of cytological atypia. Low grade pancreatic intraepithelial neoplasia 1B simulates lesions of pancreatic intraepithelial neoplasia 1A. However, layering epithelium demonstrates papillary or micro-papillary architecture or basal epithelial pseudo-stratification (Tables 1 & 2).
Low grade pancreatic intraepithelial neoplasia 2 exhibits flattened to papillary mucinous epithelial proliferations. Nuclear anomalies as loss of polarity, nuclear enlargement, nuclear crowding, hyperchromatic nuclei or pseudostratified nuclei are focal or minimal. Mitotic figures are exceptional. Apical or atypical mitosis are absent. High grade pancreatic intraepithelial neoplasia 3 or carcinoma in situ predominantly enunciates papillary or micro-papillary architecture. Exceptionally, flattened epithelial lesions are discerned. Cribriform or tufting pattern and luminal necrosis appears indicative of pancreatic intraepithelial neoplasia 3. Cytological examination of aforesaid lesions delineates loss of polarity, dystrophic mucinous cells, enlarged, irregular nuclei and prominent or macro- nucleoli. Mitotic activity is significant. Atypical mitosis may be discerned. Carcinoma in situ lesions is contemplated to be high grade pancreatic intraepithelial neoplasia 3. Low grade pancreatic intraepithelial neoplasia is configured of pancreatic intraepithelial neoplasia 1A, pancreatic intraepithelial neoplasia 1B and pancreatic intraepithelial neoplasia 2 (Figures 1 & 2).
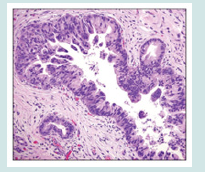
Figure 1: Pancreatic intraepithelial neoplasia demonstrating flattened and micro-papillary lesions of mucus secreting columnar epithelium with few mitotic figures abutting foci of invasive pancreatic ductal adenocarcinoma [5].
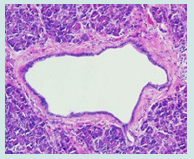
Figure 2: Pancreatic intraepithelial neoplasia delineating papillary, micro-papillary and cribriform architecture of mucus secreting glandular epithelium lining pancreatic ducts surrounded by unremarkable pancreatic stroma [6].
Pancreatic Intraepithelial Neoplasia 1A is configured of flattened epithelial lesions layered with tall columnar epithelial cells with miniature, spherical to elliptical, basal nuclei and abundant supranuclear mucin. Elliptical nuclei appear oriented perpendicular to basement membrane. Significant histological concurrence between non neoplastic, flattened, hyperplastic lesions and flattened neoplastic lesions devoid of atypia is encountered. Therefore, aforesaid lesions may be designated as the modifier lesion pancreatic intraepithelial neoplasia low grade 1A(‘PanIN/[L]-1A’). Thus, it may be surmised that the neoplastic nature of pancreatic intraepithelial neoplasia 1A remains unestablished. Pancreatic Intraepithelial Neoplasia 1B is comprised of low-grade epithelial lesions exemplifying papillary or micro-papillary lesions or lesions with basal pseudo-stratification. Besides, architecture of lesions is reminiscent of lesions encountered in pancreatic intraepithelial neoplasia1A .
Pancreatic Intraepithelial Neoplasia 2 is comprised of mucinous epithelial lesions demonstrating flat or papillary architecture. By definition, lesions necessitate occurrence of cogent nuclear abnormalities such as loss of polarity, nuclear crowding, enlarged nuclei, pseudo-stratification and hyperchromatic nuclei. However, discernible nuclear abnormalities fail to adhere to categorical anomalies encountered within nuclei of pancreatic intraepithelial neoplasia 3. Mitoses is exceptional and confined to the non-luminal, non-apical segment of pancreatic epithelium. Atypical mitotic figures are absent. True cribriform architecture, luminal necrosis and marked cytological abnormalities are absent. Pancreatic Intraepithelial Neoplasia 3 or high grade pancreatic intraepithelial neoplasia demonstrate papillary or micro-papillary configuration. Exceptionally, lesions may appear flattened. True cribriform architecture, budding off of miniature cellular clusters of epithelial cells into ductal lumen, significant cytological abnormalities or luminal necrosis appear indicative of high-grade lesion. Upon cytological assessment, lesions delineate characteristic features of loss of nuclear polarity, dystrophic goblet cells with goblet cell nuclei oriented towards duct lumen and mucinous cytoplasm oriented towards basement membrane. Several mitotic figures, along with atypical mitosis, irregular nuclei and prominent or macro-nucleoli may be discerned.
Pancreatic intraepithelial neoplasia is immune reactive to MUC5AC, MUC6, non-breast variant of HER2, fascin, MUC1 or cyclin D1. Ki67 labelling index appears elevated with enhancing grade of pancreatic intraepithelial neoplasia. A subset of highgrade lesions overexpress p53 or loss of SMAD4 / DPC4 gene. Pancreatic intraepithelial neoplasia is immune non-reactive to p63 or p40. Pancreatic intraepithelial neoplasia requires segregation from neoplasms such as normal mucosal elements and peri-biliary glands in ampulla and bile ducts, transitional metaplasia, squamous metaplasia, cancerization of ducts, tumoral intraepithelial neoplasia, simple mucinous cysts or vascular invasion in pancreatic ductal adenocarcinoma [3,4]. Low grade pancreatic intraepithelial neoplasia demonstrates inconsequential prognostic outcomes. High grade lesions are accompanied by significant, proportionate progression into pancreatic ductal adenocarcinoma and mandate meticulous monitoring and evaluation. Irrespective of grade, pancreatic intraepithelial neoplasia appearing within surgical margin of resected pancreas harboring foci of invasive carcinoma appears devoid of cogent prognostic significance. However, high grade pancreatic intraepithelial neoplasia type 3 or carcinoma in situ confined to surgical margin of resected pancreas in the absence of invasive carcinoma necessitates meticulous assessment [5,6].
References
- Tanji Y, Furukawa K, Shirai, Haruki K, Onda S, et al. (2022) Pancreatic intraepithelial neoplasia with carcinoma in situ with repeated distally localized pancreatitis: a case report. Surg Case Rep 8(1):
- Longnecker Daniel S, Suriawinata Arief A (2022) Incidence of Pancreatic Intraepithelial Neoplasia in an Autopsy Series. Pancreas 51(4): 305-309.
- Padma Kadiyala, Eileen Carpenter, Ahmed Elhossiny, Yaqing Zhang, Sarah Nelson, et al. (2022) Investigating the role of pancreatic intraepithelial neoplasia in development of pancreatic ductal adenocarcinoma. Cancer Res 82(22 Suppl): C062.
- Keiji Hanada, Akihiro Shimizu, Keisuke Kurihara, Morito Ikeda, Takuya Yamamoto, et al. (2022) Endoscopic approach in the diagnosis of high-grade pancreatic intraepithelial neoplasia. Dig Endosc 34(5): 927-937.
- Figure 1 Courtesy: John Hopkins Pathology.
- Figure 2 Courtesy: Research gate.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...