Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Research Article(ISSN: 2641-1652) 
Acute Upper Gastrointestinal Bleeding (UGIB) In A Resource Limited Setting Highly Endemic for Viral Hepatitis B: Which Etiologies for Which Real Clinical Practices? Volume 3 - Issue 2
Firmin Ankouane1,2*, Mathurin Pierre Kowo1,3, Antonin Wilson Ndjitoyap Ndam1,4, Winnie Bekolo5,6, Angèle Nadège Odjolo7, Raïssa Flore Tsakem Esobze7, Dominique Noah Noah5,8, Servais Albert Fiacre Eloumou Bagnaka5,9
- 1Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon.
- 2Yaounde Central Hospital, Cameroon.
- 3University Teaching Hospital Yaoundé, Cameroon.
- 4Yaoundé General Hospital, Cameroon.
- 5Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Cameroon.
- 6Douala General Hospital, Cameroon.
- 7University of the Mountains, Banganté, Cameroon.
- 8Laquintinie Hospital Douala, Cameroon.
- 9Gynaeco-Obstetric and Paediatric Hospital Douala, Cameroon.
Received:June 09, 2021 Published: June 24, 2021
*Corresponding author:Firmin Ankouane, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon.
DOI: 10.32474/CTGH.2021.03.000157
Abstract
Aim : To determine the aetiologies of acute upper gastrointestinal bleeding (UGIB) in a setting highly endemic for hepatitis B and to describe actual clinical practices in a resource-limited setting.
Patients and methods: This study was conducted in two parts. The first part was retrospective from January 1st 2010, to December 31st 2019 on the epidemiological profile of UGIB and the second was a prospective study from December 1st 2017 to May 31st 2018 to evaluate, in a blinded experiment, the actual clinical practices in front of an acute UGIB at the emergency units in Yaounde (Cameroon), and included: recognizing UGIB, assessing for severity, taking emergency measures and prescribing emergency Eosogastroduodenal endoscopy (EGDE).
Results : During the retrospective period, 506 patients (prevalence of acute UGIB in the services 5.6%) were included of which 71.3% were men (sex ratio 2.5). The mean age was 49.9 +/- 8 years. Haematemesis was inaugural in 350 patients (69.1%), nonsteroidal anti-inflammatory drugs were the main risk factor in 297 (43.6%), in 78 (15.4%), this was a second episode. Clinical parameters showed initial instability in 435 patients (85.9%) and haemoglobin (Hb) was <7g/dl in 359 (83.4%). EGDE was performed in 203 patients (40.2%), the main causes of UGIB were lesions of portal hypertension in 111 (44.7%), followed by peptic ulcers in 108 (43.5%). Treatment was mainly medical. However, 94 patients (84.7%) with portal hypertension lesions received endoscopic treatment, mainly by injection of sclerosing agent (69.1%), as well as 13 (1.2%) with peptic ulcers, mainly by isolated injection of dilute adrenaline (1: 10,000) in 11 (84.6%). A total of 75 patients (14.8%) died. The second part concerned 74 patients admitted for acute UGIB at the emergency services of five hospitals in Yaounde. To recognize UGIB, a digital rectal examination was done in 43 patients (58.1%), no patient received a nasogastric tube. For assessment of severity, blood pressure was taken in 73 patients (98.6%), pulse rate in 61 (82.4%), respiratory rate in 17 (23%), saturation in 17 (23%), no patient had prognostic scores in their record. For resuscitation measures, 10 patients (13.5%) received a double peripheral venous line, 20 (27%) were filled with crystalloids, restrictive blood transfusion (Hb < 7 g /dl) was carried out in 24 out of 27 patients (88.9%), 9 (12.2%) received nasal oxygen therapy. EGDE was carried out in 43 patients (60.6%), all beyond 24 hours and none had a prognostic score (Forrest or Rockall).
Conclusion: Rupture of oesogastric varices plays a significant role in the occurrence of UGIB in areas with high hepatitis B endemicity, with exceptional severity and high mortality among young people. The lack of qualified human resources and insufficient technical facilities constitute a serious problem. Locally applicable protocols are needed. In the long term, eliminating viral hepatitis B and C should reduce the prevalence of UGIB
Keywords:Gastrointestinal Bleeding; Hepatitis B Virus; Portal Hypertension; Limited Resources; Endoscopy; Clinical Practice.
Background
Acute gastrointestinal bleeding is one of the major medical and surgical emergencies whose severity should never be underestimated [1]. In approximately 80% of cases, acute gastrointestinal bleeding is of high origin, i.e. the aetiology of the bleeding is located upstream of the duodenojejunal angle or Treitz angle [2]. Acute upper gastrointestinal bleeding (UGIB) is the most frequent emergency in hepato-gastroenterology and remains a major cause of mortality, despite improvements in technical facilities, the mortality rate remains stable at about 10-15% [1-4]. The UGIB is exteriorized in 66% of cases in the form of hematemesis and the aetiologies involved are varied [2,5-7]. In the West, the proportion of peptic ulcers is significantly high. Indeed, the most common causes of acute UGIB are non-varicose (80-90%) and include gastric and duodenal ulcers in 20-50% [2,3,5-7]. Contrarily, in sub-Saharan Africa, the proportion of portal hypertension lesions is significant [8,9]. The impact of chronic hepatitis B virus (HBV) infection in this highly endemic area is significant. In highly endemic countries, ≥8% HBsAg positivity, the HBV-related disease burden is due to liver cancer and cirrhosis in adulthood, responsible for portal hypertension. The majority (80%) of the world population lives in high- or intermediate-endemic areas [10]. The way to handle acute UGIB is well codified. Gastrointestinal bleeding must be recognised, its severity assessed, and blood loss compensated. Finally, the cause of the bleeding must be found and treated [1,2,7,11]. The diagnostic approach, non-specific measures to prevent or treat haemorrhagic shock and specific haemostasis measures according to the aetiology of UGIB are often not all implemented in resources limited countries and this has an impact on evolution and prognosis. Based on data collected in the files of patients admitted in emergency and those obtained following the daily clinical practice of emergency staff, the study aimed to highlight the epidemiology and actual management of acute UGIB in our context dominated by HBV infection and limited resources.
Methods
A retrospective collection of data contained in the files of patients admitted for acute UGIB at the Yaounde Central Hospital (Cameroon) between January 1st2010 and December 31st2019, was carried out. Located at the heart of the city of Yaounde, the Yaounde Central Hospital (YCH) was created in 1930. It is the largest public referral hospital in Cameroon with capacity of …. Beds that also acts as a teaching hospital. It houses several services including the Hepato-gastroenterology service with a capacity of 34 beds, three university specialists, four general practitioners and several permanent workers and residents. This service has an upper and lower gastrointestinal endoscopy room and endoscopy equipment. The variables recorded included: demographics (age, sex); clinical and biological characteristics (onset of UGIB, history of bleeding, physical parameters of bleeding severity, and haemoglobin levels on admission); Esogastroduodenal endoscopy (EGDE) findings; specific management of the cause of the bleeding and outcomes (Table1).
Table 1: Clinical and biological parameters of 506 patients admitted at the Yaounde Central hospital for a recognized acute UGIB.
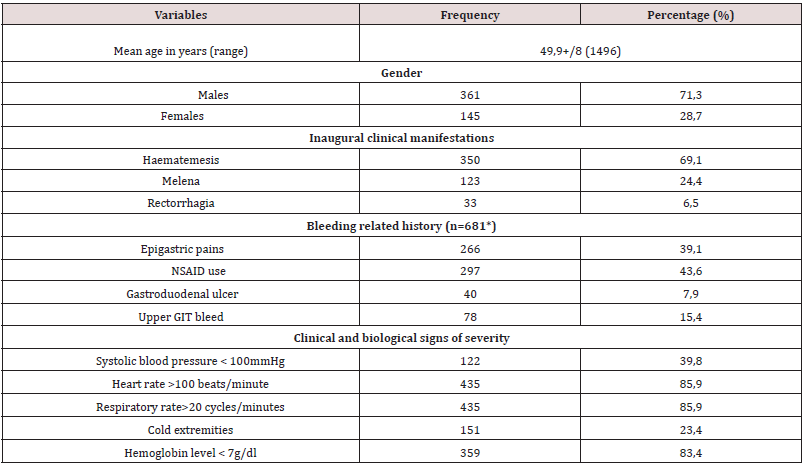
Table 2: Main causes of acute UGIB at the Yaounde Central Hospital amongst 203 patients who had an EGD.
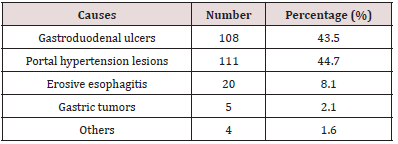
The second component, cross-sectional and observational blinded experiments, conducted from December 1st 2017 to May 31st 2018, assessed the diagnostic approach and actual management of patients admitted for acute UGIB in five hospitals of different categories (1st to 3rd) of the Cameroonian health pyramid, in the city of Yaounde. Twenty (20) doctors and 39 nurses in the emergency service who managed 74 patients admitted for acute UGIB were followed. The elements to take care of acute UGIB included recognition of acute UGIB by placing a nasogastric tube or performing a digital rectal exam; assessment of severity by clinical criteria (taking blood pressure (BP), pulse rate (PR), respiratory rate (RR) and room oxygen saturation (SPO2), Glasgow- Blatchford score; fluid resuscitation and transfusions (double venous line, crystalloids filling, proton pump inhibitors (PPI) or vasoactive treatment, oxygenation, restrictive blood transfusion (haemoglobin (Hb) <7 g/dl), hourly monitoring of vital signs and neurological status) and finally, the performance of EGDE and the Rockall and Forrest scores.
Statistical Analysis
Data was analysed using Statistical Package for Social Sciences (SSPS Inc, Chicago, Illinois, USA) version 23.0. Means ± standard deviation was used for quantitative variables; Categorical data was expressed as numbers and proportions. A p value of less than 0.05 was considered statistically significant.
Results
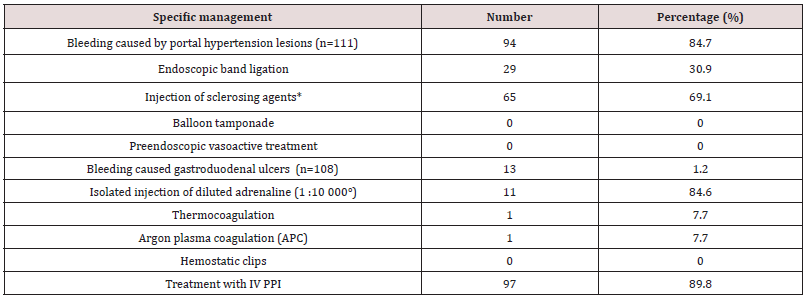
During the retrospective period, 506 patients (prevalence of acute UGIB in the services 5.6%) were included of which 361 men (71.3% and 145 women (28.7 %), given a 2.5 sex ratio. The mean age was 49.9+/- 8 years (maximum 14-96 years) and the peak of bleeding was in the 55-65-year age group. Haematemesis was the initial complaint in 350 patients (69.1%), non-steroidal antiinflammatory drugs (NSAIDs) constituted the most important risk factor in 297 patients (43.6%), in 78 (15.4%). This was a second episode. On admission, clinical parameters relevant to severity were systolic BP <100 mmHg in 122 patients (39.8%); HR >100 beats/ minutes in 435 (85.9%) and RR >20 cycles/minute in 435 (85.9%). Hb was <7g/dl in 359 patients (83.4%). EGDE was performed in 203 patients (40.2%), the major causes of bleeding were: portal hypertension lesions in 111 patients (44.7%) followed by peptic ulcers in 108 (43.5%). Treatment was mainly medical. However, 94 patients (84.7%) with portal hypertension lesions received endoscopic treatment, mainly by injection of sclerosing agent (69.1%), as well as 13 patients (1.2%) with peptic ulcers, mainly isolated injection of dilute adrenaline (1: 10,000) in 11 (84.6%). A total of 75 patients (14.8%) died during hospitalization (Table 2).
The second part concerned the assessment of care offered to 74 patients admitted for acute UGIB (mean age 55 years; sex ratio 2.1). For the recognition of bleeding, digital rectal examination was performed in 43 patients (58.1%) and no patient received nasogastric tube. Regarding the assessment of severity by clinical criteria, BP was taken in 73 patients (98.6%), HR in 61 (82.4%), and RR in 17 (23%), no patient had prognostic scores in the record, including the Glasgow-Blatchford score. Only 17 patients (23%) had SPO2 measurements. Regarding intensive care measures, only 10 patients (13.5%) received a double peripheral venous line, the majority of which was a small-bore venous line. Twenty patients (27%) were filled with crystalloids; restrictive blood transfusion was performed in 24 out of 27 patients (88.9%) with Hb < 7 g / dl. Only 9 patients (12.2%) received nasal oxygen therapy. EGDE was performed in 43 patients (60.6%), all beyond 24 hours after admission and none had a prognostic score after endoscopy (Forrest or Rockall).
Discussion
The study showed that acute UGIB is an emergency with exceptional severity in our environment, as mortality is very high at around 15%. In this study, it was found that acute UGIB affected two and a half times more men than women with a mean age of about 50 years. This is the case in studies conducted in Mali (sex ratio 2.78; mean age 47.45 years), and in Côte d’Ivoire (sex ratio 3.38; mean age 47 years) [8,12]. Indeed, in the sub-Saharan African region, the occurrence of acute UGIB often involves relatively young patients. This can be explained by the fact that the causes of bleeding are often dominated by portal hypertension lesions, especially in young patients, as reported in Mali in a rural area [8]. As opposed to sub-Saharan Africa, in the West, the age of onset of acute UGIB is higher than 70 years due to the use of NSAIDs in the elderly population. Of chronic NSAID users, 25% develop an ulcer, of which 2-4% are complicated by bleeding [2,7,13,14]. The male predominance is universal, and reverses in the West after the age of 80 years due to the higher life expectancy in the female population [2,4,6-9, 11,13,14].
As in several studies reported in literature, hematemesis was the most common initial clinical presentation [2,6,7,15,16]. This initial clinical presentation can be explained by the different lesions found on endoscopy. In fact, portal hypertension lesions, led by oesophageal varices, were the most frequent, alongside peptic ulcers. The frequency of the various aetiologies varies from one region to another [5,6,8]. Thus, in the sub-Saharan African region, several studies report very high frequencies of portal hypertension lesions. This is the case of the study by Diarra et al. in Mali in 2007 [8]. The authors reported a frequency of 55.2% in favour of ruptured oesophageal varices, far ahead of peptic ulcers which represented 16%. The frequency of portal hypertension lesions is also high in Burundi (28.2%) [16] and Gabon (29.5%) [17]. These various countries have liver diseases related to chronic HBV infection in common. Indeed, sub-Saharan African countries are located in a zone of high endemicity according to the World Health Organisation (WHO), i.e. the prevalence of hepatitis B is 8-20% of the general population [10]. Cirrhosis, which causes portal hypertension, is often the result of chronic HBV infection acquired at birth or in early childhood [18]. The annual incidence of varicose veins is approximately 5% [19]. UGIB from ruptured esophageal varices accounts for 70% of gastrointestinal bleeding in cirrhosis, with an estimated overall 2-year bleeding risk of 20% [19]. The aetiological approach to acute UGIB in high- and intermediate-endemic areas where the majority (80%) of the world population lives should therefore consider this high frequency of portal hypertension lesions and bleeding complications.
Regarding the severity of the bleeding, more than 85% of patients were initially unstable with clinical and laboratory signs of severity, despite the absence of Glasgow-Blatchford and Rockall scores in their records. This initial haemodynamic instability can be explained, on the one hand, by the causes of bleeding, in particular the rupture of oesogastric varices, which are exceptionally serious, but also, on the other hand, by the late arrival in hospital structures due to distance, cultural considerations or difficulties of mobility in our country.
Treatment was essentially medical. PPI treatment was most often initiated on admission, even if this treatment did not always fully comply with current recommendations in Europe and Asia [2,7,20,21]. Contrarily, in cases of suspected acute UGIB related to rupture oesogastric varices, vasoactive therapy to reduce portal blood flow was never initiated on admission, in line with international recommendations, including those adapted by the European Society of Gastrointestinal Endoscopy to be applicable to resource-limited settings, including some African countries [2,7,22-24]. Patients with bleeding peptic ulcers have rarely benefited from endoscopic haemostasis, unlike those with portal hypertension lesions. Late arrival at hospital would explain why in sub-Saharan Africa Forrest scores IIc and III, i.e. pigmented stain or clean base of the ulcer, are most frequently found [25]. For the Forrest classification guiding the choice of endoscopic treatment modality for ulcers, there is no endoscopic treatment for these scores [2,3,7,26]. The practice of injecting adrenaline alone instead of combined adrenaline and bipolar coagulation or endoclips was inappropriate. This could be explained by factors such as excessive procedure costs, insufficient training of practitioners and limited logistic equipment.
The mortality rate of upper GI bleeding varies between 2-10%, and is increased by the presence of associated diseases, but also rupture of oesogastric varices with a specific mortality rate of 15- 25% [2,27,28]. Because of the predominance of portal hypertension lesions in our series and the liver failure accompanying cirrhosis, mortality was high compared to other countries in the sub-region, 5% in Togo [15], 3.8% in Gabon [17] and 9.4% in Côte d’Ivoire [12]. On the other hand, it was close to the mortality found in Burundi, 22.9% [16]. The lack of primary prevention of varicose vein rupture could also explain this high mortality. However, this factor has not been studied (Table 3, 4).
Table 4: Evaluation of diagnostic approach and intial management of 74 patients admitted for acute UGIT in 5 emergency centres in Yaounde.
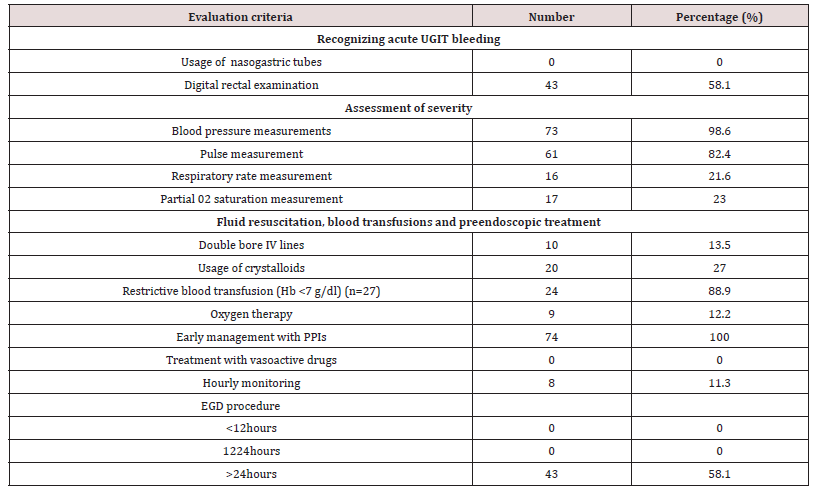
The quality of care is key to improving prognosis [2,3,7, 8,29- 31]. The actual care in the studied setting was assessed. The priority in the care of acute UGIB is to ensure haemodynamic stability with crystalloid or balanced infusions and possible transfusions [2- 5,29-31]. For this purpose, bleeding should be recognised either by nasogastric tube placement or by digital rectal examination. Also, the severity should be assessed by prognostic scores, in particular the Glasgow-Blatchford score (GBS), based on clinical and biological criteria, validated to identify low-risk patients with a sensitivity of 99% and a specificity of 32% [2,30,32]. From these recommendations, it was observed that the recognition of bleeding and the assessment of its severity were insufficient. Recording clinical parameters was neglected. Neither digital rectal examination nor nasogastric tube placement to aid diagnosis was rarely done if the patient was not present at the time of the blood loss. Oxygen therapy, even for initially unstable severe cases or cases with comorbidities, was often not effective. This may be due to the lack of oxygen in some hospitals or because of outdated or non-existent anaesthesia and intensive care equipment. The current guidelines, recommending a transfusion threshold of 7 g/dl [2,30,33,34], were met in about 90% of patients in this case. It should be noted that the Yaounde Central Hospital, which had the largest number of patients, has a certification for its blood bank. Treatment with venous PPI was started early in the majority of cases regardless of the suspected aetiology. Given the significant proportion of portal hypertension lesions, vasoactive drugs should be integrated into local protocols for the care of acute UGIB, since this is not currently the case. Helicobacter pylori testing and eradication is also not integrated into the care of acute UGIB in the setting. And yet, this is a validated approach [35]. Helicobacter pylori infection plays a major role in the development of ulcers in the studied environment, affecting approximately 70-80% of the population [36].
EGDE with or without endoscopic therapeutic procedures is an integral part of the care of UGIB and has been shown to improve patient outcomes in terms of morbidity and mortality [2,7,26,31]. However, the ideal time for its performance is unclear. Recommendations agree that it should be performed within 24 hours of admission [2,3,7,37]. Early endoscopy within 12 hours would not influence clinical outcomes, mortality, recurrence of bleeding or the need for surgery [20,31]. In our resource-limited environment, not only is there a shortage of qualified human resources, but there is also inadequate technical equipment. While it is accepted that an EGDE should be performed in every patient with acute UGIB, the rate of completion was only 40-60.6% in both study arms. This procedure is relatively expensive in relation to people’s income. Endoscopy was most often performed beyond 24 hours after admission. Thus, therapeutic endoscopic procedures were rare. If performed early, it seems to have an influence on hospital resources and costs, by identifying low-risk patients and allowing them to return home quickly [31,37]. This would be appropriate in our resource-limited environment.
Conclusion
Contrary to Western countries, ruptured oesogastric varices play an important role in the occurrence of gastrointestinal bleeding in areas with high hepatitis B endemicity. UGIB is exceptionally severe in these areas, as it has a high mortality. The lack of qualified human resources and insufficient technical facilities constitute a serious problem. For this reason, the improvement of clinical practice in relation to acute UIGB should consider the significance of portal hypertension lesions, on the one hand, and the limitation of resources on the other. Therefore, locally applicable protocols need to be devised. In the long term, the elimination of viral hepatitis B and C should considerably reduce the prevalence of UGIB. A multicentre study is therefore expected to establish protocols adapted to our health and limited resource settings.
What Is Known
a) 80% of gastrointestinal bleeding are upper. The most common causes of acute UGIB are non-variceal (80-90%) and include gastric and duodenal ulcers in 20-50%.
b) The age of onset of UGIB is around 70 years.
c) UGIB remains an important cause of morbidity and mortality.
The mortality rate has remained stable at around 10-15%.
d) The improvement of the prognosis depends on the care quality and treatment of acute UGIB is well codified.
e) The assessment of the severity of UGIB is based on prognostic scores, in particular the Glasgow-Blatchford score (GBS) which is based on clinical and biological criteria, validated to identify low-risk patients with a sensitivity of 99% and a specificity of 32%.
f) If acute UGIB is suspected in relation to rupture oesogastric varices, vasoactive therapy to reduce portal blood flow should be given as early as possible. In cases of suspected ulcerrelated UGIB, high-dose PPI therapy should be started early.
g) The priority in the care of acute UGIB is to ensure haemodynamic stability with crystalloid or balanced infusions and possible transfusions. Current guidelines recommend a transfusion threshold of 7 g/dl.
h) Helicobacter pylori testing and eradication is incorporated into the care of acute HDH.
i) It is accepted that an EGDE should be performed in all patients with acute UGIB. There is consensus that endoscopy should be performed within 24 hours of admission (12 hours in the case of portal hypertension related UGIB).
j) The Forrest classification guides the choice of endoscopic treatment modality for bleeding ulcers.
k) Haemostatic endoscopy is the first-line treatment for bleeding ulcers.
Relevance of the study
a) The causes of acute UGIB in areas of high hepatitis B endemicity are dominated by variceal bleeding in young patients.
b) The age of onset of UGIB is around 50 years.
c) The mortality rate is very high, around 15%.
d) The actual clinical practice is based on a non-codified care.
e) The prognostic scores of UGIB, notably those of Glasgow- Blatchford (GBS), Forrest and Rockall, are rarely used.
f) In case of suspected acute UGIB related to rupture oesogastric varices, vasoactive treatment is not often prescribed.
g) Not all emergency intensive care measures are followed, including oxygen therapy and monitoring.
h) Helicobacter pylori testing and eradication is not included in the care of acute UIGB.
i) The rate of completion of EGD is very low (40-61%) and it is done beyond the required 24 hours.
j) Haemostatic endoscopy is not the first-line treatment for bleeding ulcers, only in 1.2%.
Abbreviations and acronyms:
Upper gastrointestinal bleeding (UGIB); hepatitis B virus (HBV); HBV surface antigen (HBsAg); blood pressure (BP); pulse rate (PR), respiratory rate (RR); room oxygen saturation (SPO2), oesogastroduodenal endoscopy (EGDE); haemoglobin (Hb); proton pump inhibitors (PPIs); University of the Mountains (UdM); World Health Organisation (WHO); Institutional Ethics Committee of the University of the Mountains (CIE-UdM).
Declarations
a) Ethical considerations
We obtained ethical clearance from the Institutional Ethics Committee of the University of the Mountains (CIE-UdM) under the number No. 2018/149/UdM/PR/CIE of 19 March 2018.
b) Consent to publish
All authors gave their approval for publication.
c) Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
d) Competing interests
The authors declare no conflict of interest for this study.
e) Funding
The authors did not receive any fund for this study.
References
- Frexinos J, Buscail L, Staumont G (2004) Acute gastrointestinal bleeding. In: For the practitioner, hepato-gastroenterology. 5th Paris Masson p.25-34.
- Weiss E, Paugam Burtz C (2016) Gastrointestinal bleeding. Anesthesia & Resuscitation. 2: 292-299.
- Romailler E, Frossard JL, Carballo S (2019) Optimal and endoscopic management in upper gastrointestinal bleeding. Rev Med Switzerland 15: 1859-1864.
- Khamaysi I, Gralnek IM (2013) Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract Res Clin Gastroenterol 27: 633-638.
- Kamboj AK, Hoversten P, Leggett CL (2019) Upper Gastrointestinal Bleeding: Etiologies and Management. Mayo Clin Proc 94: 697-703.
- Patteron D, Chaillet M, Debuc E (2007) Gastrointestinal haemorrhages. National Congress anesthesia and reanimation 2007 the essentials pp. 477-486.
- Lesur G, Camus M (2015) How to optimize the endoscopic management of upper digestive haemorrhages? Acta Endosc 45: 151-155.
- Diarra M, Soucko Diarra A, Dolo M (2007) Acute upper gastrointestinal bleeding: Experience from a rural setting. Acta Endosc 37: 321-326.
- Ankouane Andoulo F, Ngo Nonga B, Noah Noah D (2013) Aetiology and risk factors of acute upper gastrointestinal bleeding: analysis of 613 cases in Yaounde, Cameroon. Port Harcourt Med J 7: 175-182.
- World Health Organization (2017) Global hepatitis report 2017.
- Okon AB, Thot'o A, Diakité M (2015) Results and predictors of mortality from upper gastrointestinal bleeding in hospitalization: multicenter study in Côte-d'Ivoire. Acta Endosc 45: 285-290.
- Bouglé A, Harrois A, Duranteau J (2008) Management of hemorrhagic shock in resuscitation: principles and practices. Resuscitation 17(2): 153-161.
- Garza González E, Perez Perez GI, Maldonado Garza HJ (2014) A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 20: 1438-1449.
- Lanza FL, Chan FK, Quigley EM (2009) Practice Parameters Committee of the American College of Gastroenterology Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 104: 728-738.
- Bagny A, Bouglouga O, Djibril MA (2012) Etiological profile of upper gastrointestinal hemorrhages in adults at the Lomé University Hospital-Campus (Togo). J Afr Hepato Gastroenterol 6: 38-42.
- Ntagirabiri R, Mumana A, Dunduri D (2012) Upper gastrointestinal bleeding in adults in Burundi: epidemiological, etiological, therapeutic and evolutionary aspects. J Afr Hepato Gastroenterol 6: 272-275.
- Itoudi Bignoumba PE, Maganga Moussavou IF, Moussavou Kombila JB (2019) Upper Digestive Hemorrhage at the Center Hospitalier Universitaire de Libreville: Clinical Aspects and Actual Management: About 210 Patients. Health Sci Dis 20: 20-22.
- World Health Organization / UNICEF estimate of national immunization coverage 2014, WHO-UNICEF(2015).
- D’Amico G, Luca A (1997) portal hypertension. Natural history. Clinical-hemodynamic correlations. Prediction of the risk of bleeding. Baillere’s Clin Gastroenterol 11: 243-256.
- Gralnek IM, Stanley AJ, Morris AJ (2021) Endoscopic diagnosis and management of nonvariceal upper gastrointestinal bleeding (NVUGIB): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy 53: 300-332.
- Kim JS, Kim BW, Kim DH (2020) Guidelines for nonvariceal upper gastrointestinal bleeding. Gut Liver 14: 560-570.
- De Franchis R, Baveno VIF (2015) Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratyfying risk and individualizing care for portal hypertension. J Hepatol 63: 743-752.
- Hassan C, Aabakken L, Ebigbo A (2018) Partnership with African Countries: European Society of Gastrointestinal Endoscopy (ESGE)-Position Statement. Endosc Int Open 6: E1247-E1255.
- Karstensen JG, Ebigbo A, Bhat P (2020) endoscopic treatment of variceal upper gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) cascade guideline. Endosc Int Open 08: E990-E997.
- Akere A, Osundina MA (2017) Upper gastrointestinal bleeding: forrest classification and rebleeding in patients with peptic ulcer disease at teaching hospital in southwest Nigeria. Nigerian J Gastroenterol Hepatol 9: 25-34.
- Cook DJ, Guyatt GH, Salena BJ (1992) Endoscopic therapy for acute nonvariceal upper gastrointestinal bleeding: a meta-analysis. Gastroenterology 102:139-48.
- Bouglouga O, Bagny A, Lawson Anissoh L (2014) Hospital mortality linked to upper gastrointestinal bleeding by rupture of an oesophageal varicose vein at the University Hospital of Lomé (Togo). Med Health Trop 24: 388-391.
- Cales P, Pascal JP (1988) Natural history of esophageal varices in cirrhosis (from birth to rupture). Gastroenterol Clin Biol 12: 245-254.
- Nable JV, Graham AC (2015) Gastrointestinal Bleeding. Emerg Med Clin North Am 2016; 34:309-25. Simon TG, Travis AC, Saltzman JR. Initial assessment and resuscitation in nonvariceal upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am 25: 429-442.
- Simon TG, Travis AC, Saltzman JR (2015) Initial Assessment and Resuscitation in Nonvariceal Upper Gastrointestinal Bleeding. Gastrointest Endosc Clin N Am 25: 429-442.
- Cai JX, Saltzman JR (2018) Initial assessment, risk stratification, and early management of acute nonvariceal upper gastrointestinal hemorrhage. Gastrointest Endosc Clin N Am 28: 261-275.
- Blatchford O, Murray WR, Blatchford M(2000) A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 356: 1318-1321.
- Villanueva C, Colomo A, Bosch A (2013) Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 368: pp.2341.
- Zhang W, Zheng Y, Yu K (2021) Liberal transfusion versus restrictive transfusion and outcomes in critically ill adults: A Meta-Analysis. Transfus Med Hemother 48: 60-68.
- Barkun AN, Bardou M, Kuipers EJ(2010) International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 152: 101-13.
- Ankouane Andoulo F, Noah Noah D, Tagni Sartre M (2013) Epidemiology of Helicobacter Pylori infection in Yaoundé: from the peculiarity to the African enigma. Pan Afr Med J 16:pp. 115.
- Tsoi KK, Ma TK, Sung JJ (2009) Endoscopy for upper gastrointestinal bleeding: how urgent is it? Nat. Rev Gastroenterol Hepatol 6: 463-469.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...