Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6062
Case Report(ISSN: 2638-6062) 
What do we know now about the Relationship Between Early Repolarization and Malignant Ventricular Arrhythmia? About a Case Report Volume 4 - Issue 3
Fiama Caimi Martinez1, Guido Antoniutti1, Rocio Blanco1, Jorge Alvarez Rubio1,7, Santiago Magnani Raganato1, Gabriela Ávila velazquez2, Laura Torres Juan3, Ana Belén García2, Consuelo Pérez Luengo2, Susana Moyano4, Juan Carlos Canós4, Alexandra Ladino4, Elena Hernández Marin5, Estela García5, Nieves Sánchez2, Gloria Gutiérrez2, Lorena Diaz4, Juan Carlos Borondo4, Manuel Crespillo5, Catalina Melià1,7 and Bernardino Barceló6,7, Damian Heine Suñer3,7 and Tomas Ripoll Vera1,7,8*
- 1Inherited Heart Disease Unit. Hospital Universitario Son Llatzer 07198 Palma de Mallorca, Spain
- 2Legal Medicine Institute of the Balearic Islands, Palma de Mallorca, Spain
- 3Molecular Diagnostics and Clinical Genetics Unit. Hospital Universitario Son Espases, Spain
- 4Histopathology Unit. Forensic Sciences and Toxicology Nacional Institute. Barcelona Spain
- 5Chemistry Unit Forensic Sciences and Toxicology Nacional Institute. Barcelona, Spain
- 6Clinical Analysis Laboratory. Hospital Universitario Son Espases. Palma de Mallorca, Spain
- 7Health Research Institute of the Balearic Islands (IdISBa) Palma de Mallorca, Spain
- 8CIBER of physiopathology of Obesity and Nutrition, Madrid, Spain
Received: May 12, 2022; Published: June 07, 2022
*Corresponding author: Tomas Ripoll Vera, Inherited Heart Disease Unit. Hospital Universitario Son Llatzer, Health Research Institute of the Balearic Islands (IDISBA), CIBER of physiopathology of Obesity and Nutrition, Madrid, Spain
DOI: 10.32474/PRJFGS.2019.03.000190
Abstract
After observing a case of Sudden Death (SD) of unexplained cause with an electrocardiographic pattern of Early repolarization (ERP), we set out to demonstrate the characteristics that re-late this entity to the reports of SD, in order to clarify which patients are at higher risk of malignant arrhythmic events.
Keywords: Sudden Death; Early Repolarization; Autopsy; J-Wave Syndromes
Introduction
The prevalence of ERP in the general population ranges from 3% to 24% according to the different registries, with a predominance in the male sex. Likewise, in Western countries, up to 2% of all SD are secondary to arrhythmic disorders, among which are the different channelopathies, such as long QT syndrome, short QT syndrome, catecholaminergic polymorphic ventricular tachycardia and J-wave syndromes: Brugada Syndrome (BrS) and Early repolarization Syndrome (ERS) [1]. ERP has been observed in up to 33 15% of cases of SD due to idiopathic ventricular fibrillation, especially in people aged 35 to 45 years [2,3]. The study of the relationship between ERP and SD has increased exponentially in the last 10 years, in an attempt to identify its pathophysiological mechanism and its role in triggering ventricular arrhythmia [4,5].
Case Reportn
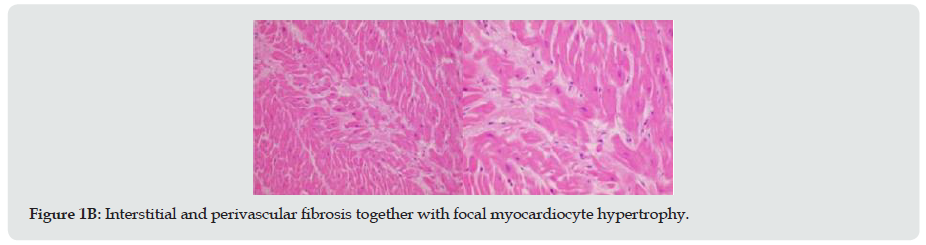
The patient was a 38-year-old male smoker with no personal or family history of pathology. He lived with his wife and children, whom he saw at night before going to bed, without symptoms. In the early morning he was found lifeless by his relatives, who called the emergency services. He was in bed in the prone position, the emergency services did advance cardiopulmonary resuscitation, but after 30 minutes of unsuccessful manoeuvres the resuscitation was discontinued, and death was established. As there was no apparent cause of death and the patient was under 40 years of age, the case was classified as sudden unexplained death syndrome (SUDS) and was included in the Balearic Islands Sudden Death Protocol (MUSIB) for study. The MUSIB is a programme for the study of patients under 50 years of age who died of non-violent sudden death, through post-mortem studies including molecular autopsy by massive next generation sequencing (NGS), followed by a cascade family study in cases that merit it, in the territory of the Balearic Islands [6]. An autopsy was performed, and the heart was found to weigh 468 g. Macroscopically, it did not appear to present valvular or coronary artery lesions. The only relevant histopathological finding was mild interstitial and perivascular subendocardial fibrosis and focal myocyte hypertrophy. After partial study of the main structures of the conduction system, partial fibrosis of the sinus node was identified (Figure 1). The rest of the autopsy was without pathological findings.
As part of the protocol, a toxicological examination was performed, on which tetrahydrocannabinol was detected in the blood and urine at low concentrations. Genetic analysis of 118 genes related to SUDS using NGS did not reveal any pathogenic genetic variants (Table 1). The results of previous cardiological studies were requested, revealing an electrocardiogram (ECG) in sinus rhythm, AQRS +60, p 80 msec, PR 120 msec, QRS 80 msec, ascending st segment suprathreshold in inferior and anterior face (Figure 2). Based on the recognition of an ERP on the ECG, we decided to extend the genetic panel to include KCND2 and DPP10, which were also normal. Subsequently, the family group was evaluated in cascade, studying both parents, two sisters and two children, showing no apparent structural or arrhythmogenic heart disease, including ERP, except for a case of sudden infant death in a third-degree relative (Figure 3).
Figure 4: Electrocardiogram of the deceased 1 year prior to sudden death. Sinus rhythm, AQRS +60, p 80 msec, PR 120 msec, QRS 80 msec, ascending st segment suprathreshold in inferior and anterior face.

Figure 5: Family tree: The index patient is shown with an arrow. ERS affected and sudden death. Not affected E1 Echocardiogram: (- negative)/ (+ positive) E2 ECG (- negative)/ (+ positive).

Discussion
Unexplained SD syndrome is defined as a death occurring in an individual older than 1 year with negative pathological and toxicological evaluation, and cardiac SD when death occurs within 1 hour of the onset of symptoms in witnessed cases, and within 24 hours after the last time the individual was seen alive when the death was not witnessed [7]. The cause of SD is cardiac in 53% and unexplained in 24% of cases, of which up to 70% present some genetic variant that suggests the aetiology of the SD, a fact that demonstrates the value of molecular study [6,8]. On the other hand, reports of ERP date back to 1936, when Shipley and Hallaran described ERP as a variant of normality [9]. Since then, several dissimilar definitions of this entity have been published, describing its value as a differential diagnostic pattern of other entities, which in the absence of precordial pain, was interpreted as a variant of normality [10,12]. In 1984, Otto, et al. [13] identified the abnormal transition of the STQRS segment as a possible marker of increased arrhythmic risk; in turn, in 1993, a Japanese group described the presence of notching (notch at the end of the QRS) in patients with idiopathic ventricular fibrillation [14,15]. Only in 2008 did Haïssaguerre et al. demonstrate, using a registry of patients with sudden death events, the presence of J wave slurring (QRS elongation) and notching. In these terms demonstrate ERP regardless of the ST-segment elevation for diagnosis, as a novel observation [16]. In 2011, a classification of ECG patterns and their association with ventricular arrhythmia was published, designating patterns as ERP or BrS according to the region of the heart responsible for the arrhythmogenic substrate. This interprets these patterns as representing a spectrum of phenotypic expression under the denomination of J-wave syndromes [17].
The classifications are as follows:
a) Type 1: ERP exclusively in lateral precordial leads, high prevalence in healthy athletes, and associated with a low risk of arrhythmic events.
b) Type 2: ERP present in inferior or inferolateral leads, associated with a moderate level of risk.
c) Type 3: ERP in inferior, lateral and right precordial leads, associated with the highest level of arrhythmic risk and electrical storm.
d) Type 4: Electrocardiographic pattern limited to right precordial leads, corresponding to BrS.
This classification has been widely criticised due to its lack of pathophysiological support to explain it (Figure 4). ERP is defined if the following electrocardiographic pattern is recognized: (1) final QRS notch (J wave) on the downslope of a prominent R wave, with or without ST-segment elevation; (2) peak notch or J wave ≥0.1 mV in ≥2 contiguous 12-lead ECG leads, excluding leads V1– V3; and (3) QRS duration (measured in leads in which there is no notch or ligature) of 120 ms. This fact decentralized the ST-segment suprathreshold to the J point as the focus. On the other hand, ERS was defined in those patients meeting the Shanghai criteria, which was developed later (Figure 5) [4].
Pathophysiology
The current understanding of the pathophysiological mechanism of ERP is incomplete. It is likely that an intraventricular transmural voltage gradient or dispersion between the epicardium and endocardium occurs during the action potential, which manifests as a J wave on the surface ECG [18,20]. Electrical heterogeneity results in phase 2 re-entries, creating an arrhythmogenic surface capable of provoking ventricular arrhythmias [21]. In turn, reasons for the pathophysiological differences between groups are postulated; for example, the increased frequency in the male sex could be attributed to higher testosterone levels relative to women, resulting in abnormal potassium ion channel current and increased J-point elevation. Other differences between population groups remain poorly understood in terms of their pathophysiological basis, such as the higher prevalence of PR in smokers and athletes compared to the general population [22,24]. Parallel to the description of the electrical basis of the pathogenicity of early repolarization, it is unknown whether this phenomenon coexists with structural heart disease. In this regard, the coexistence of fibrosis with BrS has been described, with which it shares the denomination of J-wave syndromes. However, it does not present significant interactions with respect to the location of fibrosis or the compromised tissue layer. More evidence regarding structural heart disease is essential in order to obtain an objective tool for follow-up and risk stratification and to demonstrate a conclusive relationship [25]. Therefore, it is not possible to conclude that there is a relationship between conduction system fibrosis and ERS in the case presented. Regarding the genetic basis of this disease, the current knowledge is limited to case reports, in which KCNJ8, ABCC9, CACNA1C, CACNB2, CACNA2D1, ANK3, lSCN5A and SCN10A, and with lower prevalence, KCNd2 and DPP10, are most frequently involved [26,30]. The evidence is limited and based mainly on case reports, with limited and poor demonstration of familial segregation.
Prognosis
At first, ERP was catalogued as an arrhythmic risk factor in patients with coronary artery disease, in whom the prevalence is higher compared to the general population (20.1% vs. 6.2%), with a higher rate of arrhythmic events [31,33]. Subsequently, a Finnish registry associated ERP with arrhythmic risk in patients with non-ischemic cardiac SD, where one in five SD victims (20.7%) presented an ECG with an inferolateral ERP pattern, compared to 5.3% in the general population [34]. On the other hand, other studies have reported a higher incidence of SD events in patients younger than 50 years with an ERP, compared to those older than 50 years, with no differences with respect to sex, ethnicity, or family history of ERP [35,38]. Based on these data, the Shanghai score has been developed in order to clarify which patients with an ECG pattern of ER are at high risk of SD through clinical variables, ECG, ambulatory ECG monitoring, family history and genetic testing. This score classifies as ERS those patients with at least one ECG finding meeting and with 5 or more points [4]. Our presented case meets 5 points in order to define it as ERS associated with SD of unexplained cause. On the other hand, there are other variables not considered in the Shanghai score that are considered to be of prognostic value:
a) Notch in QRS
b) Coexistence with short QT syndrome or BrS
c) Increased J wave amplitude, increased in response to sympathetic stimulation
d) Persistent pattern in exercise testing
e) Electrophysiological provocation study with abnormally short activation-recovery intervals in the inferior and lateral ventricular regions in patients with ERP, the substrate of ventricular arrhythmias [39,44]. It is possible that certain conditions that predispose a patient to repolarization heterogeneity, such as ST-elevation myocardial infarction, sympathetic hyperactivation, hypokalemia and heart failure, increase the arrhythmogenic potential of ERP [45].
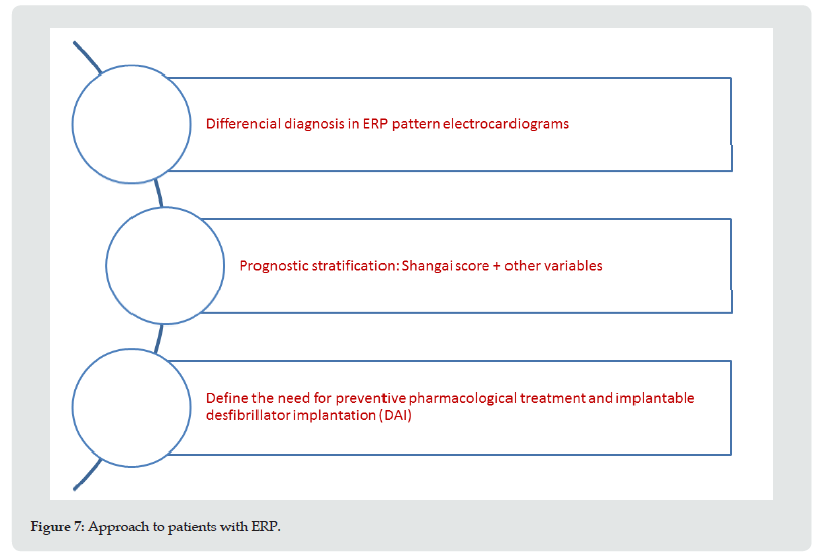
Undoubtedly, there is sufficient evidence to link the presence of ERP with an arrhythmogenic substrate, independently of the underlying pathophysiology [46]. Through evaluation based on the Shanghai score and other prognostic variables, allows us, through our interpretation, to classify patients into groups using the following approach (Figure 6). The starting point in the diagnosis of the ERP pattern is the differential diagnosis of entities that present with ST-segment elevation, such as ischemic heart disease, pericardial syndromes and BrS [47-49]. Secondly, there is the classification as ERS using the Shanghai score, with the intention of identifying its prognostic stratification. Finally, the need for preventive pharmacological treatment and implantation of a subcutaneous holter and clinical follow-up or cardio defibrillator (ICD) is assessed. There are no reports regarding the usefulness of other techniques, such as radiofrequency ablation (Figure 7).
Medical treatment with drugs such as quinidine or hydroquinidine has been shown to achieve long-term suppression of ventricular fibrillation and ventricular tachycardia, although the evidence is limited and discordant. In the case of other antiarrhythmic drugs such as amiodarone, mexiletine, verapamil, beta-blockers, and class IC drugs, they have shown no or very modest results in the prevention of arrhythmias in this patient population. Finally, extrapolating similarities with the pathophysiology of BrS, cilostazol, milrinone and isoproterenol may be able to suppress arrhythmogenesis associated with ERP, although large-scale randomized studies are needed in order to demonstrate this [50,51]. Regarding treatment for the secondary prevention of J-wave syndromes, the indication for ICD implantation is clear, and in primary prevention it is described in
i. Patients with recovered SD or documentation of ventricular tachycardia (VT)/ ventricular fibrillation (VF), with or without syncope (Class I).
ii. Patients with symptoms of syncope, nocturnal agonal breathing and family history of sudden death at an early age, presumably of arrhythmic aetiology (Class IIb).
iii. Asymptomatic patients with a high-risk electrocardiographic pattern and family history of sudden death at an early age, presumably of arrhythmic aetiology (Class IIb) [4].
Conclusion
There is a probable relationship between SD, ERP and conduction system fibrosis in the clinical case presented, given the various variables and their relationship with malignant ventricular arrhythmia in the presence of SUDS without other aetiological data explaining his death. While there are few patient registries concerning ERP, the high prevalence in the general population and low rate of serious arrhythmic events, coupled with a lack of specificity in the determination of arrhythmic risk, underlie the practical difficulty for prognostic stratification. Future clinical and experimental studies should focus on long-term prognostic estimation, emphasising the genetic origin and relationship with structural heart disease, and evidence of fibrosis.
Supplementary Materials
Not applicable.
Author Contributions
Conceptualization
Fiama Caimi Martinez, Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio,Tomas Ripoll Vera.
Methodology
Fiama Caimi Martinez, Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Catalina Melià Mesquid, Tomas Ripoll Vera.
Software
Fiama Caimi Martinez, Guido Antinuitti, Rocio Blanco.
Validation
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Gabriela Ávila Velázquez, Laura Torres Juan, Belén García, Maria Ines Fernández Sierra, Sara Sánchez Peira,Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela, Albert Vingut López, Elena Hernández Marin, Estela García García, Bernardino Barceló, Damian Heine Suñer, Catalina Melià Mesquida, Tomas Ripoll Vera.
Formal Analysis
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Gabriela Ávila Velázquez, Laura Torres Juan, Belén García, Maria Ines Fernández Sierra, Sara Sánchez Peira, Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela, Albert Vingut López, Elena Hernández Marin, Estela García García, Bernardino Barceló, Damian Heine Suñer, Catalina Melià Mesquida, Tomas Ripoll Vera.
Investigation
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Laura Torres Juan, Catalina Melià Mesquida, Tomas Ripoll Vera.
Resources
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Gabriela Ávila Velázquez, Laura Torres Juan, Belén García, Maria Ines Fernández Sierra, Sara Sánchez Peira, Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela, Albert Vingut López, Elena Hernández Marin, Estela García García, Bernardino Barceló, Damian Heine Suñer, Catalina Melià Mesquida, Tomas Ripoll Vera.
Data Curation
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Gabriela Ávila Velázquez, Laura Torres Juan, Belén García, Maria Ines Fernández Sierra, Sara Sánchez Peira, Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela, Albert Vingut López, Elena Hernández Marin, Estela García García, Bernardino Barceló, Damian Heine Suñer, Catalina Melià Mesquida, Tomas Ripoll Vera.
Writing-Original Draft Preparation
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Tomas Ripoll Vera.
Writing-Review and Editing
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Gabriela Ávila Velázquez, Laura Torres Juan, Belén García, Maria Ines Fernández Sierra, Sara Sánchez Peira, Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela, Albert Vingut López, Elena Hernández Marin, Estela García García, Bernardino Barceló, Damian Heine Suñer, Catalina Melià Mesquida, Tomas Ripoll Vera.
Visualization
Fiama Caimi Martinez,Guido Antoniutti, Rocio Blanco, Jorge Alvarez Rubio, Juan Carlos Borondo Alcázar, Susana Moyano Corvillo, Juan Carlos Canós Villena, Concepcion Dasi Martínez. Alexandra Ladino Orjuela.
Supervision
Jorge Alvarez Rubio,Tomas Ripoll Vera.
Project Administration
Tomas Ripoll Vera
*All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of “Comité 240 Ético de Investigación Clinica de Les Illes Balears” (protocol code IB 1525/11PI and date of approval 23/2/2011) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgment
CIBEROBN CB12/03/30038.247.
Conflicts of Interest
The authors declare no conflict of interest.
References
- William J McKenna, Daniel P Judge (2021) Epidemiology of the Inherited Cardiomyopathies. Nature Reviews Cardiology 18(1): 22-36.
- Haruta Daisuke, Kiyotaka Matsuo, Akira Tsuneto, Shinichiro Ichimaru, Ayumi Hida et al. (2011) Incidence and Prognostic Value of Early Repolarization Pattern in the 12-Lead Electrocardiogram. Circulation 123(25): 2931-2937.
- Sayedahmad Ziad, Fahed Darmoch, Yasser Al Khadra, Amjad Kabach, M Chadi Alraies (2019) Does Early Repolarization on ECG Increase the Risk of Cardiac Death in Healthy People? Cleveland Clinic Journal of Medicine 86(3): 165-166.
- Antzelevitch Charles, Gan-Xin Yan, Michael J Ackerman, Martin Borggrefe, Domenico Corrado, et al. (2017) J-Wave Syndromes Expert Consensus Conference Report: Emerging Concepts and Gaps in Knowledge. Europace 19(4): 665-694.
- Macfarlane Peter W, Charles Antzelevitch, Michel Haissaguerre, Heikki V Huikuri, Mark Potse, et al. (2015) The Early Repolarization Pattern. Journal of the American College of Cardiology 66 (4): 470-477.
- Ripoll-Vera Tomás, Consuelo Pérez Luengo, Juan Carlos Borondo Alcázar, Ana Belén García Ruiz, Nieves Sánchez Del Valle, et al. (2021) Sudden cardiac death in persons aged 50 years or younger: diagnostic yield of a regional molecular autopsy program using massive sequencing. Spanish journal of cardiology 74(5): 402-413.
- Stiles Martin K, Arthur A M Wilde, Dominic J Abrams, Michael J Ackerman, Christine M Albert, et al. (2020) 2020 APHRS/HRS Expert Consensus Statement on the Investigation of Decedents with Sudden Unexplained Death and Patients with Sudden Cardiac Arrest, and of Their Families. Heart Rhythm 18(1): e1-e50.
- Iglesias Mercedes, Tomas Ripoll-Vera, Consuelo Perez-Luengo, Ana Belen García, Susana Moyano, et al. (2021) Diagnostic Yield of Genetic Testing in Sudden Cardiac Death with Autopsy Findings of Uncertain Significance. Journal of Clinical Medicine 10(9): 1806.
- Shipley RA, Hallaran WR (1936) The Four-Lead Electrocardiogram in Two Hundred Normal Men and Women. American Heart Journal 11(3): 325-345.
- Wasserburger Richard H, William J Alt (1961) The Normal RS-T Segment Elevation Variant. The American Journal of Cardiology 8(2): 184-192.
- Kambara Hirofumi John Phillips (1976) Long-Term Evaluation of Early Repolarization Syndrome (Normal Variant RS-T Segment Elevation). The American Journal of Cardiology 38(2): 157-166.
- Klatsky Arthur L, Rudolph Oehm, Robert A Cooper, Natalia Udaltsova, Mary Anne Armstrong (2003) The Early Repolarization Normal Variant Electrocardiogram: Correlates and Consequences. The American Journal of Medicine 115(3): 171-177.
- Otto Catherine M, Tauxe RV, Greene HL, Gross BW, et al. (1984) Ventricular Fibrillation Causes Sudden Death in Southeast Asian Immigrants. Annals of Internal Medicine 101(1): 45-47.
- Patton Kristen K, Patrick T Ellinor, Michael Ezekowitz, Peter Kowey, Steven A Lubitz, et al. (2016) Electrocardiographic Early Repolarization: A Scientific Statement From the American Heart Association. Circulation 133(15): 1520-1529.
- Biasco Luigiet, Yvonne Cristoforetti, Ole De Backer, Davide Castagno, Carla Giustetto, et al. (2016) Early Repolarization: An Evolving Concept for the Past 70 Years. Journal of Cardiovascular Medicine 17(1): 4-10.
- Haïssaguerre Michel, Nicolas Derval, Frederic Sacher, Laurence Jesel, Isabel Deisenhofer,et al. (2008) Sudden Cardiac Arrest Associated with Early Repolarization. New England Journal of Medicine 358(19): 2016-2023.
- Antzelevitch Charles, Gan-Xin Yan (2011) J-Wave Syndromes. From Cell to Bedside. Journal of Electrocardiology 44(6): 656-661.
- Gussak Ihor, Charles Antzelevitch (2000) Early Repolarization Syndrome: Clinical Characteristics and Possible Cellular and Ionic Mechanisms. Journal of Electrocardiology 33(4): 299-309.
- Ali Abdi, Nida Butt, Azeem S Sheikh (2015) Early Repolarization Syndrome: A Cause of Sudden Cardiac Death. World Journal of Cardiology 7(8): 466-475.
- Obeyesekere Manoj N, George J Klein, Stanley Nattel, Peter Leong-Sit, Lorne J Gula, et al. (2013) A Clinical Approach to Early Repolarization. Circulation 127(15): 1620-1629.
- Rezus Ciprian, Floria M, Moga VD, Sirbu O, Dima N, et al. (2014) Early Repolarization Syndrome: Electrocardiographic Signs and Clinical Implications: Early Repolarization Syndrome». Annals of Noninvasive Electrocardiology 19(1): 15-22.
- Barra S, Providência R, Paiva L, Nascimento J (2013) Early Repolarization Patterns and the Role of Additional Proarrhythmic Triggers. Europace 15(4): 482-485.
- Felix B, Denis A, Cheniti G, Lam A, Vlachos K, et al. (2018) Early Repolarization Syndrome: Diagnostic and Therapeutic Approach. Front in Cardiovasc Med 5: 169.
- Tada H, Tadokoro K, Ito S, Naito S, Hashimoto T, et al. (2007) Idiopathic Ventricular Arrhythmias Originating from the Tricuspid Annulus: Prevalence, Electrocardiographic Characteristics, and Results of Radiofrequency Catheter Ablation. Heart Rhythm 4(1): 7-16.
- Miles C, Asimaki A, Ster IC, Papadakis M, Gray B, et al. (2021) Biventricular Myocardial Fibrosis and Sudden Death in Patients With Brugada Syndrome». J Am Coll Cardiol 78(15): 1511-1521.
- Haïssaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, et al. (2009) Ventricular Fibrillation with Prominent Early Repolarization Associated with a Rare Variant of KCNJ8/K ATP Channel. J Cardiovasc Electrophysiol 20(1): 93-98.
- Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpón E, Dan Hu, et al. (2010) Mutations in the Cardiac L-Type Calcium Channel Associated with Inherited J-Wave Syndromes and Sudden Cardiac Death. Heart Rhythm 7(12): 1872-1882.
- Perrin MJ, Adler A, Green S, Al-Zoughool F, Doroshenko P, et al. (2014) Evaluation of Genes Encoding for the Transient Outward Current (Ito) Identifies the KCND2 Gene as a Cause of J-Wave Syndrome Associated With Sudden Cardiac Death. Circulation: Cardiovascular Genetics 7(6): 782-789.
- Barajas-Martinez H, Hu D, Pfeiffer R, Burashinkov E, Powers A, et al. (2012) A Genetic Variant in DPP10 Linked to Inherited J-Wave Syndrome Associated with Sudden Cardiac Death by Augmentation of Kv4.3 Channel Current. Heart Rhythm 9(11): 1919-1920.
- Steinfurt Johannes, Bezzina C, Biermann J, Staudacher D, Marschall C, et al. (2021) Two Siblings with Early Repolarization Syndrome: Clinical and Genetic Characterization by Whole-Exome Sequencing. EP Europace 23(5): 775-780.
- Fan J, Feng-Juan Yao, Yun-Jiu C, Cheng-Cheng J, Xu-Miao C, et al. (2020) Early Repolarization Pattern Associated with Coronary Artery Disease and Increased the Risk of Cardiac Death in Acute Myocardium Infarction. Annals of Noninvasive Electrocardiology 25(6): e12768.
- Yoshihisa, Naruse Y, Tada H, Harimura Y, Ishibashi M, et al. (2014) Early Repolarization Increases the Occurrence of Sustained Ventricular Tachyarrhythmias and Sudden Death in the Chronic Phase of an Acute Myocardial Infarction. Circulation: Arrhythmia and Electrophysiology 7(4): 626-632.
- Saagar M, Sacher F, Berte B, Yamashita S, Lim H (2014) Evaluation of Patients With Early Repolarization Syndrome. Journal of Atrial Fibrillation 7(3): 1083.
- Holmström LTA, Haukilahti MA, Tikkanen JT, Aro AL, Kenttä TV, et al. (2018) Inferolateral Early Repolarization among Non-Ischaemic Sudden Cardiac Death Victims. EP Europace 20(F11): f93-f98.
- Holkeri A, Eranti A, Haukilahti MAE, Kerola T, Kenttä TV, et al. (2020) Impact of Age and Sex on the Long-Term Prognosis Associated with Early Repolarization in the General Population. Heart Rhythm 17: 621-28.
- Raphael R, Adler A, Halkin A, Viskin S, et al. (2011) Risk of Sudden Death among Young Individuals with J Waves and Early Repolarization: Putting the Evidence into Perspective. Heart Rhythm 8(6): 923-929.
- Saagar M, Derval N, Sacher F, Berte B, Yamashita S, et al. (2015) History and Clinical Significance of Early Repolarization Syndrome. Heart Rhythm 12(1): 242-249.
- Yun-Jiu C, Xiao-Xiong L, Cheng-Cheng Ji, Xu-Miao C, Li-Juan L, et al. (2016) Role of Early Repolarization Pattern in Increasing Risk of Death. Journal of the American Heart Association 5(9): e003375.
- Haïssaguerre M, Nademanee K (2019) Depolarization versus Repolarization Abnormality Underlying Inferolateral J-Wave Syndromes: New Concepts in Sudden Cardiac Death with Apparently Normal Hearts. Heart Rhythm 16(5): 781-790.
- Anne R, Maury P, Bongard V, Sacher F, Delay M, et al. (2012) Prevalence, Prognosis, and Identification of the Malignant Form of Early Repolarization Pattern in a Population-Based Study. The American Journal of Cardiology 110(9): 1302-1308.
- Toledano K, Rozin AP (2013) Early Repolarization: Innocent or Dangerous?. The American Journal of the Medical Sciences 346(3): 226-232.
- Nam GB, Kwan-Ho Ko, Kim J, Kyoung-Min P, Kyoung-Suk R, et al. (2010) Mode of Onset of Ventricular Fibrillation in Patients with Early Repolarization Pattern vs. Brugada Syndrome. European Heart Journal 31: 330-339.
- Watanabe Hiroshi, Makiyama T, Koyama T, Kannankeril PJ, Seto S, et al. (2010) High Prevalence of Early Repolarization in Short QT Syndrome. Heart Rhythm 7(5): 647-652.
- Palmiere Cristian, Maria del Mar Lesta, Jessica Vanhaebost, Patrice Mangin, Marc Augsburger, et al (2014) Early Repolarization, Acute Emotional Stress and Sudden Death. Journal of Forensic Sciences 59(3): 836-840.
- Junttila M Juhani, Jani T Tikkanen, Tuomas Kenttä, Olli Anttonen, Aapo L Arol, et al (2014) Early Repolarization as a Predictor of Arrhythmic and Nonarrhythmic Cardiac Events in Middle-Aged Subjects. Heart Rhythm 11(10): 1701-1706.
- Tikkanen Jani T (2014) The Phenomenon of Early Repolarization: A False Alarm? Circulation: Arrhythmia and Electrophysiology 7(3): 368-369.
- Yan Gan-Xin (2016) MY APPROACH to Early Repolarization Syndrome. Trends in Cardiovascular Medicine 26(4): 393-394.
- Derval Nicolas, Frederic Sacher, Arnaud Denis, Michel Haïssaguerre (2016) Management of an Asymptomatic Patient with Dynamically Changing J Wave from Inferior Early Repolarization to Brugada Pattern. Heart Rhythm 13(2): 565-568.
- Nam GB, Kim YH, Antzelevitch C (2008) Augmentation of J waves and electrical storms in patients with early repolarization. The New England journal of medicine 358(19): 2078-2079.
- Adler Arnon, Michael H Gollob (2015) A Practical Guide to Early Repolarization. Current Opinion in Cardiology 30(1): 8-16.
- Di Diego JM, Antzelevitch C (2018 J wave syndromes as a cause of malignant cardiac arrhythmias. Pacing and Clinical Electrophysiology 41(7): 684-699.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...