Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6062
Research Article(ISSN: 2638-6062) 
Standardization of Synchronization Procedure to Collect the Similar Aged C. elegans Volume 4 - Issue 1
Sarita Mishra, Aaditya Vikram Gaur and Rakhi Agarwal*
- Laboratory of Analytical & Molecular Toxicology (Forensic Chemistry & Toxicology Laboratory), Institute of Forensic Science, Gujarat Forensic Sciences University, India
Received: February 28, 2020; Published: March 05, 2020
*Corresponding author: Rakhi Agarwal, Laboratory of Analytical & Molecular Toxicology (Forensic Chemistry & Toxicology Laboratory), Institute of Forensic Science, Gujarat Forensic Sciences University, Sector 09, Gandhinagar, 382007, Gujarat India
DOI: 10.32474/PRJFGS.2020.04.000178
Abstract
The selection of synchronized stage (same age group) is needed in any model organism viz worm, fly, zebrafish, those are having different developmental stages such as embryonic, larval, pupal then adult. Thus, synchronization is the key for the success of any toxicological experiments. C. elegans has been popularly used for the assessment of toxicity associated with pesticides and several other environmental toxicants. C. eleganslife cycle consists of embryonic stage, four larval stages and then adult stage. Thus, obtaining the similar age group worms will be helpful for different developmental, behavioural, and neuronal toxicity studies. Though, several synchronization protocols are already available however, they are complicated, time consuming, results in lesser yield and with more animal debris but in the present study we have shown a simplified, detailed protocol to collect the synchronized worms using swinging block rotors for centrifugation purpose.
Keywords: Synchronization C; Elegans; Bleach Treatment; Gravid Adult; Centrifugation with Swinging Block Rotors
Background
Caenorhabditis elegansis one of the popular experimental model organisms because of its ease of culturing, transparency, short life cycle and homology to human genome [1]. C. elegans develops through various embryonic and 4 larval stages to an adult [1-3]. Culturing of worms results in presence of variable age group population in the media. Synchronous population is very much needed for eliminating the variation due to age differences. Synchronization in terms of development is to be of the same age at the same time with respect to other members of the group [4]. Several assays for toxicological assessment which are age dependent require the worms of same age group [5]. Age synchronization of worms involves lysis of the gravid animal body by using axenizing solution to liberate the eggs. Previously available age synchronization protocol requires more time and efforts, resulted in lesser yield along with more accumulation of animal debris[6,7]. The present detailed protocol has simplified the process,and has resulted in better yield along with minimum unwanted animal debris by using swinging block rotors. The current protocol will be helpful to collect the synchronized worms for conducting the studies where experimental outcome can be affected by age-differences of the animal/worms [8-10]. This will be helpful to examine the adverse effects of various pesticides/toxicants exposure to the large population of similar aged worms.
Materials and Reagents
a. Sodium hypochlorite solution (5%, Sigma-Aldrich, CAS
No. 7681-52-9)
b. 5N NaOH, (SRL chemicals, CAS No. 1310-73-2, Mumbai,
India)
c. M9 buffer
Equipment
a. Refrigerated Centrifuge with swinging block rotors
(Thermofisher Scientific, Model-Heraeus Megafuge 8R)
b. Laminar air flow (Thermo scientific, HERAGUARD ECO)
c. Pipettes (P1000)
d. Compound microscope (Micros, Austria)
e. 15ml flacon tube (Genaxy)
f. 90×15 mm petri plate (Genaxy)
Procedure
a) For synchronization of worm, take plate containing maximum gravid adult C. elegans (Figure.1).
Figure 1: (A) Image showing gravid hermaphrodite C. elegans.
(B) Magnified image of gravid C. elegans showing eggs inside the body.
(C) Disintegration of C. elegans body after bleach treatment and release of the egg.

b) Hold the plate at an angle of 45°, add 1 ml of M9 buffer
(3gms KH2PO4/L, 6gms Na2HPO4/L, 5gms NaCl/L, 1ml of 1M
MgSO4/L) and swirl gently in order to dislodge worms, collect
the worm wash in 15ml falcon tube by simply decanting it.
c) Add 1ml of M9 buffer on the same plate and collect the
worm wash in the same 15ml falcon tube.
d) Repeat the step 3 again.
e) Pour 500μl M9 buffer, swirl gently and collect in the same
15ml falcon tube.
f) Add freshly prepared 1.5ml axenizing solution (1ml
Sodium hypochlorite + 0.5ml 5 N NaOH) to the same falcon
tube.
g) Tap gently with finger for exactly 7 mins.
h) Centrifuge at 3410 rpm for 30 secs at 22 °C using swinging
block rotors.
i) Remove the supernatant carefully leaving behind the
pellet in falcon tube.
j) Add 5-7 ml of M9 buffer in order to wash off axenizing
solution from the surface of egg pellet.
k) Tap gently with fingers for exactly 2 mins.
l) Centrifuge at 3410 rpm, for 30 secs at 22 °C using swinging
block rotors.
m) Discard the supernatant leaving behind the pellets
containing eggs in flacon tube.
n) Pour pellets on to the unseeded plate and leave them for
10-12 hrs at 22oC.
o) Worms will get arrested at L1 stage after hatching.
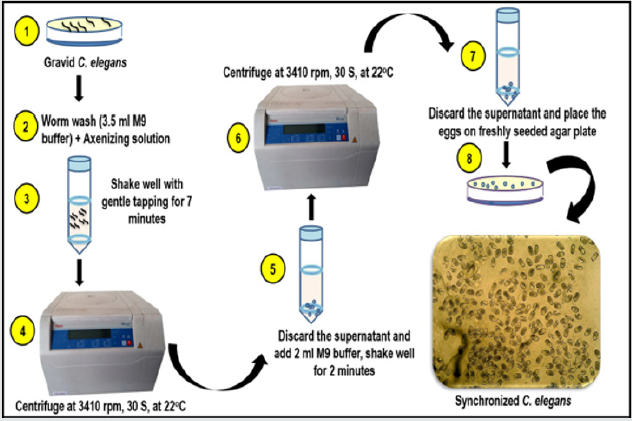
Figure 2: Age-synchronization of C. elegans-Schematic diagram showing steps involved in age synchronization of C. elegans.
p) Transfer synchronized worms onto a new freshly seeded
plate either by worm picking or by taking the worm wash.
Figure 2 is showing schematic diagrammatic representation of
steps involed in synchronization procedure.
Note
a) Plate containing gravid adult C. elegans, M9 buffer, 5N
NaOH, Sodium hypochlorite Solution should be at 22 °C at the
time of synchronization.
b) Mixing of 5N NaOH (to be prepared in autoclaved distilled
water) and sodium hypochlorite solution should be done
freshly, just before starting the synchronization and should be
mixed properly by vortexing.
c) Decreasing the rpm will give more animal debris on plate
while increasing will give less number of eggs. So be careful
with the time and rpm of centrifugation.
d) Animal should not be kept in axenizing solution more
than 7 mins otherwise eggs will also get affected. Be quick for
centrifugation and discarding the supernatant.
e) Using swinging block rotors while centrifugation gives
better result as compare to fixed axis rotation.
Recipes
For NGM Plate Preparation
1. 3 gms NaCl/L
2. 2.5 gms Peptone/L
3. 17 gms Agar/L
Measure appropriate amount of chemicals and make up the
final volume as 975 ml with distilled water.
4. 1M Cholesterol (1ml)
5. 1M CaCl2 (1ml)
6. 1M MgSO4(1ml)
7. 1M KH2PO4(25ml)
Note: Do not autoclave cholesterol
For M9 Buffer Preparation
1. 3 gms KH2PO4/L
2. 6gms Na2HPO4/L
3. 5gms NaCl/L
4. 1ml of 1M MgSO4/L
5. Distilled water to make the final volume 1 lit.
Autoclave this at 120 °C for 20 mins and cool. Store in fridge at
4 °C temperature
Acknowledgment
Authors acknowledge Director General, Gujarat Forensic Sciences University and Director, Institute of Forensic Science, Gujarat Forensic Sciences University for allowing to use the facilities and equipments in the study. Mr. Aaditya Vikram Gaur is also grateful to Gujarat Forensic Sciences University for providing Gujarat Forensic Sciences University Research Fellowship. Authors are also grateful to Dr. Aamir Nazir, Senior Scientist and Head, Fundamental Genomics and Molecular Toxicology Laboratory, Division of Toxicology, Council of Scientific and Industrial Research- Central Drug Research Institute (CSIR-CDRI), Lucknow, India, for generously providing C. elegans strain.
Competing interests
Authors declare that there is no competing interest.
Ethics
The study was approved by University Research Committee (No. PhD/FS/RA/005).
References
- Wood WB (1988) The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, USA.
- Stiernagle T (2006) Maintenance of Celegans,WormBook: The Online Review of C elegans Biology, USA.
- Brenner S (1974) Genetics 77(1):71-94.
- Duranton C, Gaunet F (2016) Behavioural synchronization from an ethological perspective. overview of its adaptive value 24(3).
- Porta de la Riva M, Fontrodona L, Villanueva A, Ceron J (2012) Basic Caenorhabditis elegans Methods: Synchronization and Observation. J Vis Exp 64: e4019.
- Fanglian He (2011) Synchronization of Worm. 1(7): 7-8.
- Sulston J, Hodgkin J (1988) In: The Nematode Caenorhabditis elegans, WB Wood, (Ed). New York: Cold Spring Harbor Laboratory Press p. 587.
- Solution WB (2015) Spring pp.6-8.
- Nonet M (2004) About the nematode Caenorhabdtis elegans.
- Piper Reid H (2017)The C elegans model in toxicity testing. JApplToxicol 37(1): 50-59.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




