Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6062
Research Article(ISSN: 2638-6062) 
Effect of Explant Type and Plant Growth Regulators Upon Callus Induction and Indirect Regeneration of Chickpea Volume 4 - Issue 1
Hamzeh Minaei Chenar, Danial Kahrizi* and Alireza Zebarjadia
- Department of Agronomy and Plant Breeding, Razi University, Kermanshah, Iran
Received: November 26, 2019; Published: December 16, 2019
*Corresponding author: Danial Kahrizi, Department of Agronomy and Plant Breeding, Razi University, Kermanshah, Iran
DOI: 10.32474/PRJFGS.2019.04.000177
Abstract
Today protein shortage is one of the prevalent problems in the developing countries people’s food program specially the groups with low income. But chickpea production is limited because of biotic and abiotic stresses. Traditional breeding methods for some reasons are time consuming and are not very efficient. It is necessary to improve quality and quantity of chickpea via genetic engineering and gene transfer. Chickpea regeneration is prerequisite for gene transformation. The chickpea indirect regeneration like other legumes is limited. In this study callus induction and indirect regeneration was investigated in Bivanij cultivar of chickpea that is one of the best chickpea cultivars. For this purpose, four explants including embryo, cotyledon, node and hypocotyl were used. These explants were cultured on the MS medium with the best growth regulators compositions that have been previously reported. Also, the inducted calli from each explant were cultured on special regeneration medium. At the stage of callus induction, for cotyledon, hypocotyl and node, MS medium supplemented with 0.5mg/l BAP+0.5mg/l NAA showed the highest response. The embryos response was well for all mediums. The obtained calli from cotyledon(29.36%,), embryo(37%) and node(29.66%), showed the highest response for indirect regeneration on MS medium supplemented with 2mg/l TDZ and hypocotyl calli showed the highest regeneration (16%) on MS medium supplemented with 2mg/L BAP+0.125mg/l IBA.
Introduction
Chickpea (Cicer arietinum) is one of the most important plant protein resources, and it has been used as food in different ways since ancient times. Chickpea is a member of the Fabaceae, stands second as for occupiearea (10 million ha) in the area under cultivation and third in production (7 million tons) among the cultivated pulses [1]. This plant is good source of carbohydrate (48.2-67.6%), protein (12.4-31.5%), starch (41-50%), fat (6%) and nutritionally important minerals [2]. Chickpea is an important grain legume in Indian subcontinent, West Asia, Mediterranean region, North and East Africa, Southern Europe and Central America and Australia [1]. One of the largest problems in today world is poverty of food. Because of high trophic value and wide dispersal of chickpea in the world, this crop can be considered in the developing countries people’s food program, especially the groups with low income and somewhat eliminates the problems of poverty of food, moreover different parts of chickpea can be used to feed livestock. It is able to drive more than 70% of nitrogen symbiotic dinitrogen fixation; which makes it a promising crop for “alternative agriculture” that is now attracting considerable attention in the industrialized world [1,3]. However, the production of this crop has remained low because of its susceptibility to biotic stresses like Ascochyta blight, fusarium and insect pest (Heliothis), also abiotic stresses such as saltiness, drought and cold [4]. Cultivars resistant to biotic and abiotic stresses which have better protein quality and quantity are needed. Chickpea, similar to other grain legumes, have narrow genetic base since they are essentially selfpollinated (although cross-pollination does take place, it is at very low frequency). Thus, there is the need to widen the genetic base and incorporate desirable characters [5]. Conventional breeding methods for stress resistance are often cost and time consuming, limited to lack of proper gen in gen pool and none crossing interspecies [6]. Modern biotechnology, including tissue culture, genetic engineering and genetic transformation techniques, has provided new opportunities to enhance the germplasm of crop plants [7]. The potential value of cell, organ, anther, microspore and embryo as tool for use in the plant breeding has been reported [8-10]. Routine transformation protocols are limited in chickpea. The low success has been attributed to poor regeneration ability (especially via callus) and lack of compatible gene delivery methods [11]. However, several researchers have described the in vitro callus induction of C. Arientinum [12-17].
There are two major factors that clearly inhibit efficient
transformation
1. The shoot regeneration rate is genotype-dependent and
genotype specificity affects transformation rate.
2. The very low efficiency of transformation indicates that
gene transfer via Agrobacterium infection into cut or injured
cotyledon or hypocotyls tissue hardly ever occurs for reasons
that are not well understood [18].
Material and Methods
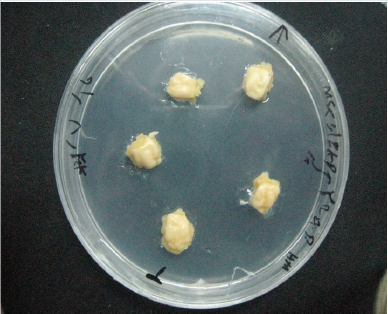
This work was conducted in the Lab Plant Tissue Culture, Agriculture and Natural Resources Campus, Razi University, Kermanshah, Iran. The seeds of chickpea (Bivanij cultivar) were obtained from Sararoud Dry Land Research Institute in Kermanshah, Iran. This cultivar is known as a high yield and sensitive to Ascochyta blight disease. Mature seeds were first screened manually, and the damaged seeds were removed. The seeds were washed with distilled water several times to clean them from any dust, then sterilized with 2% sodium hypochlorite solution for 10-15 minutes and rinsed 4-5 times in sterile distilled water and were cultured on free hormone MS medium. The seeds germinated after 3-5 days and after about 3 weeks when plantlet height was 3-5cm (Figure 1), nod and hypocotyl explants were removed carefully from stock plantlet. But to get cotyledon and embryo explants after sterilizing and rinsing, the seeds were transferred into distilled water and were soaked in sterile conditions for 24 hours. Then cotyledon and embryo were removed from seeds by use of forceps and scalpel.
Figure 1: The sterilized stock plantlets for explant preparation in order to callus induction experiment.

In this study we used basal MS (Murashige and Skoog) medium containing 3% sucrose, 0.7 agar and different combinations of plant growth regulators and the PH was adjusted to 5.7 before autoclaving. All cultures were maintained at 25±1 °C under 16hr photoperiod and with light provided by fluorescent lamps. To callus induction each explant was cultured on its special medium that have been previously reported. After callus induction, the calli could grow for 3 weeks and after that subcultured once or more (Table 1). After 5 weeks of callus induction, the obtained calli were transferred to special regeneration medium for 5-6 weeks. These mediums were the best mediums that have been previously reported too. During at this time almost all calli produced green meristemoids. For better regeneration some calli were subcultured twice or more. After few days of subcultures, some meristeomids were converted to green shoots (Table 2). Mean comparison was performed using Duncan’s Multiple Range test at 0.05 probability level after ANOVA. Statistical analyses were done using Excel 2010, SPSS Ver. 19 and SAS Ver. 9.1 software (Figures 2-4).
Table 1: Plant growth regulator compositions and concentrations for callus induction indifferent explants.
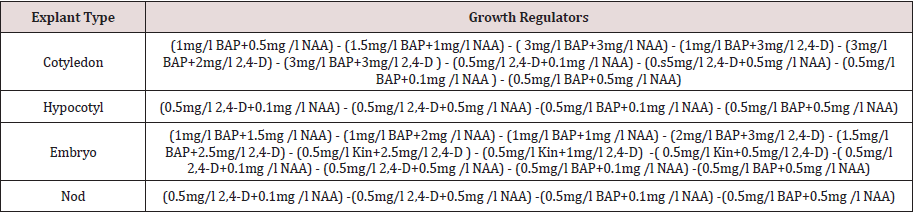
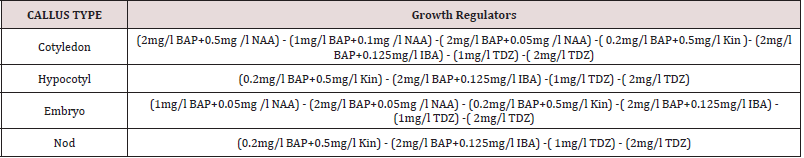
Table 2: Plant growth regulator compositions and concentrations for callus induction indifferent explants.
Results
Obtained results showed that the most PGRs had the highest callus induction percentage (about 100%) in embryo explant and the 0.1NAAmg/l+0.5mg/lBAP composition produced the lowest callus percentage (23.66%) (Figure 5). The 0.5mg/l BAP+0.5 mg/l NAA had the highest percentage of callus induction (about 95.33%) in cotyledon explant and the lowest callus produced in a concentration of (1mg/l BAP+0.5mg/l NAA) (35.00%) (Figure 6). The 0.5mg/l BAP+0.5mg/l NAA had the highest percentage of callus induction (about 70.00%) in node explant too and the (0.1 mg/l NAA + 0.5mg/l BAP) composition produced the lowest callus percentage (43.66%) (Figure 7). Results showed that the (0.5 mg/l NAA + 0.5 mg/l 2,4-D) and (0.5 mg/l BAP + 0.5 mg/l NAA) had the highest callus induction (100%) in hypocotyl explant and the (0.1mg/l NAA + 0.5mg/l 2,4-D) medium produced lowest callus percentage (64%) (Figure 8).
Figure 5: Mean comparison for plant growths regulators effects on the callus induction in embryo explants of chickpea.
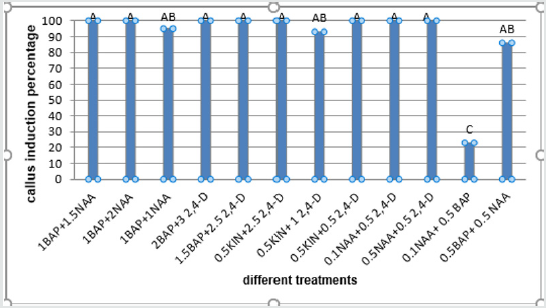
Figure 6: Mean comparison for plant growths regulators effects on the callus induction in cotyledon explants of chickpea.
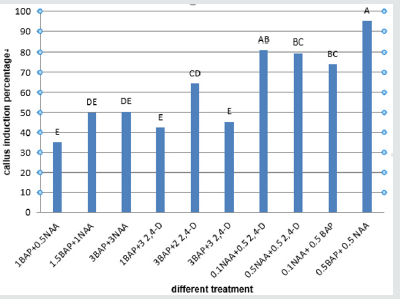
Figure 7: Mean comparison for plant growths regulators effects on the callus induction in node explants of chickpea.
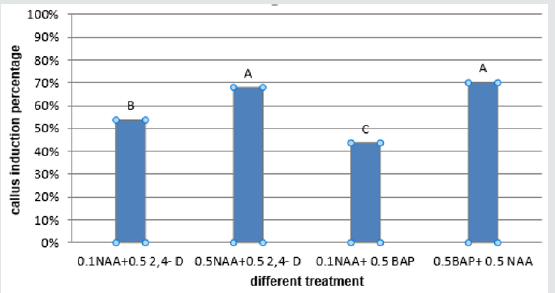
Figure 8: Mean comparison for plant growths regulators effects on the callus induction in hypocotyl explants of chickpea.
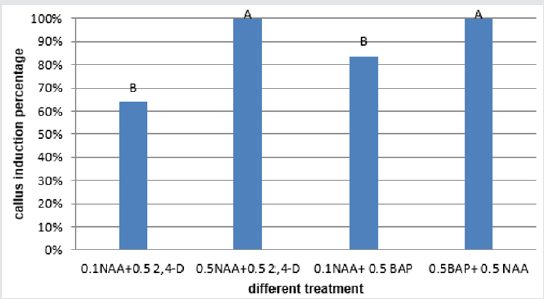
The calli of three explants including cotyledon, embryo and nod had the highest regeneration percentage in the medium supplemented with 2mg/l TDZ (29.36%, 37% and 29.66% respectively) (Figures 9-11)and for hypocotyl calli, the highest regeneration percentage was obtained in the medium supplemented by 2mg/l BAP+0.125mg/l IBA(16%) (Figure 12). Shoot regeneration percentage for calli of cotyledon, nod and hypocotyl in the medium with 0.2 mg/l BAP + 0.5 mg/l kin composition was 0% (Figures 9,11,12), and in the medium supplemented with 1 mg/l BAP + 0.05 NAA, shoot regeneration for embryo calli was 0% too [10].
Figure 9: Mean comparison for plant growths regulators effects on the shoot regeneration in cotyledon calli of chickpea.
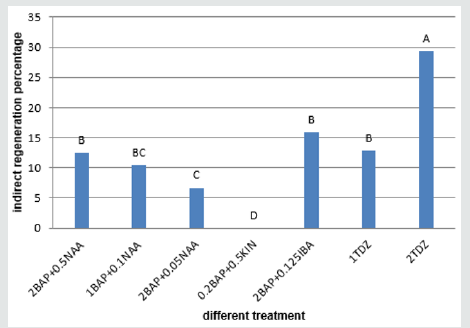
Figure 10: Mean comparison for plant growths regulators effects on the shoot regeneration in embryo calli of chickpea.
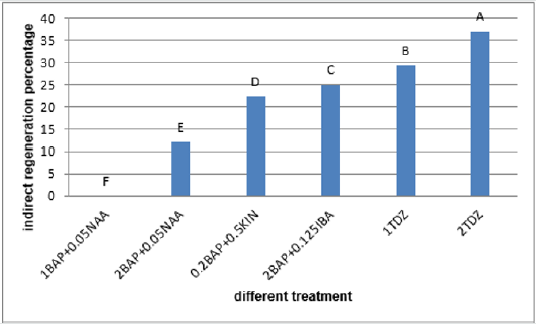
Figure 11: Mean comparison for plant growths regulators effects on the shoot regeneration in node calli of chickpea.
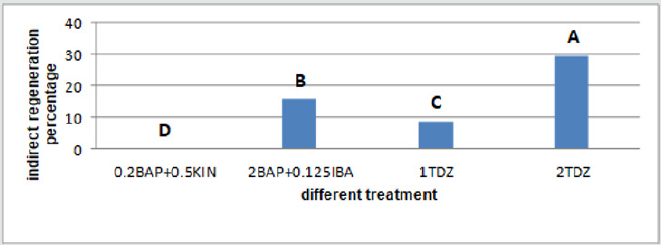
Figure 12: Mean comparison for plant growths regulators effects on the shoot regeneration in hypocotyl calli of chickpea.
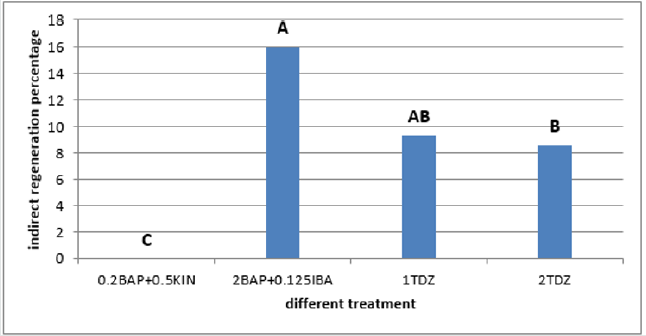
Discussion
It seems that not only 2,4-D effects on callus induction
percentage, but also increasing in NAA concentration leads to
increasing in callus induction percentage. Anju and Chawla [15]
reported that the highest callus induction percentage in chickpea
embryos was achieved in 1mg/l BAP + 1.5mg/l NAA and 1mg/l
BAP + 0.5 mg/l NAA. According to Zaman et al. [19], 100% callus
induction was achieved on 1/2MS that supplemented by 0.5 and
2.0mg/l 2,4-D that is in agreements with our results. Our results
showed that lower concentrations for 2,4-D and NAA leads to callus
induction in shorter periods. Huda et al. [20], reported that the
highest percentage for cotyledon callus induction was 95%. This
result achieved in (3mg/l 2,4-D + 3mg/l BAP) that is agreement with
our result. Difference in amount is related to difference in chickpea
genotype. There is not any report for callus induction in chickpea
node. This explant has been applied for direct regeneration [21].
It seems that hypocotyl callus induction occurs in equal amount of
auxins and Cytokinin. It may equal amounts of auxin and Cytokinin
keeps the cell in undifferentiated stage.
Neelam et al. [22], achieved the highest percentage for
hypocotyl callus induction on B5 medium supplemented by 1mg/l
BAP + 1mg/l Kin. The highest callus induction from hypocotyl
was resulted in Kaberi et al. [23], experiment on MS medium
supplemented with 2 mg/l 2,4-D + 0.5 mg/l NAA. This result is
similar to our finding.
In general, indirect shoot formation (via callus) in chickpea like
other legumes is difficult, and our work confirmed this subject.
Results of this experiment for different explants showed that callus
induction medium has important effect on indirect regeneration
(shoot induction). For example, embryo calli that had been
obtained on callus induction medium supplemented with 0.5mg/l
NAA + 1mg/l BAP had the maximum shoot regeneration, in the
regeneration medium. Arora and Chawla [24], reported that the
inducted embryo calli on the medium with 1.5mg/l NAA + 1mg/l
BAP composition, had the highest regeneration (17.6%) in the
regeneration medium with 2 mg/l BAP. Zaman et al. [19] reported
the highest regeneration for mature embryo of BARI chhola-5 in
the medium supplemented with 0.5mg/l kin + 0.5 IAA (46.15%).
Huda et al. [20], reported that the medium containing 0.5mg/l NAA
+ 2mg/l BAP had the best response (40%) in indirect regeneration
for cotyledon calli, that is similar to our finding, and difference in
amount is related to difference in chickpea genotype and culture
conditions. As aforementioned at above, there is not any report
for callus induction in chickpea node. It is noticeable that only the
green calli that produced meristemoid had shoot regeneration, thus
only these calli have capability of regeneration.
References
- Sujatha G,Jayabalan N, RanjithaK (2007) Rapid in vitro micropropagation of chickpea (CicerarietinumL). Horticultural Science34(1): 1‐5.
- Ignacimuthu S, Prakash S(2006) Agrobacterium mediated transformation of chickpea with α-amylase inhibitor gen for insect resistance. Journal of Biological Sciences 31(3): 339-345.
- Zarei I, Mohammadi G, Sohrabi Y, Kahrizi D, KhahEM, et al. (2011) Effect of different hydropriming times on the quantitative and qualitative characteristics of chickpea (Cicer arietinum L.). African Journal of Biotechnology10(66): 14844-14850.
- Kiran G, Kaviraj CP, Jogeswar G, Kavi kishor PB, Rao S(2005) Direct and high frequency somatic embryogenesis and plant regeneration from hypocotyls of chickpea (Cicer arietinum), a grain legume89(6):1012-1018.
- ZareMirakabad H, BagheriAR, ZareMehrjerdi M(2010) Efficient protocol for break impasses of regeneration via callus for 20 genotypes of Chickpea. International Journal of Plant Production 4(2): 117-130.
- Yousefiara M, Bagheri A,Moshtaghi N(2008) Optimizing regeneration condition in chickpea (Cicer arietinum L). Pakistan Journal of Biotechnology Sciences 11(7): 1009-1014.
- Sharma KK, Ortiz R (2000) Program for the application of genetic transformation for crop improvement in the semi-arid tropics. In Vitro Cellular and Developmental Biology - Plant 36(2): 83-92.
- Green CE (1977) Prospects for Crop Improvement in the Field of Cell Culture. Horticultural Science 12: 131-134.
- Vasil IK(1987) Developing Cell and Tissue Culture System for Improvement of Cereal and GrassCrops. Journal Plant Physiolgy 128: 193‐218.
- Kahrizi D, Arminian A, Masumi Asl(2011) (2ndedn),In vitroPlant Breeding Razi University Press, Iran.
- Chandra A, Pental D(2003) Regeneration and genetic transformation of grain legumes, an overview. Current Science 84: 381-387.
- Kartha KK, Pahl P, Leung NL,MroginskiLA (1981) Plant regeneration from rneristems of grain legumes: soybean, cowpea, peanut, chickpea and bean. Canadian Journal of Botany 59: 1671‐1679.
- Islam R, Riazuddin S, Farooqui H (1995) Clonal propagation from seedling nodes and shootapices of chickpea (Cicer arietinum L.). Plant Tissue Culture 5: 53‐57.
- Barna KS, WakhluAK (1994) Whole plant regeneration of Cicerarietinum from callus cultures via organogenesis. Plant Cell Report 13:510-513.
- Anju A, ChawlahS (2003) Organogenic plant regeneration via callus induction in chickpea (Cicer arietinum L.), role of genotypes, growth regulators and explants. Indian Journal Biotechnology 4:251‐256.
- Sagufta N, Aamir A, Fayyaz AS,JavedI (2008) Somatic embryogenesis from immature cotyledons and leaf calli of chickpea (Cicer arietinum L). Pakistan Journal of Botany 40(2): 523‐531.
- MinaeiChenar H, Kahrizi D,Molsaghi M(2016) Callus inductionis affected by explant type and plant growth regulators inchickpea (Cicer arietinum L.). Biharean Biologist 10(1): 28-32.
- Lee YH, Kim HS, Kim JY, Jung M, Park YS, et al.(2004) A new selection method for pepper transformation: callus-mediated shoot formation. Plant Cell Report 23(1-2): 50-58.
- Zaman MA,ManjurABMK, Ahmed M,Islam MM (2010) Effect of 2,4-D on callus induction and subsequent morphogenesis mature chickpea (Cicer arietinum L) Plant Tissue Culture and Biotechnologypp. 53-58.
- Huda S, Siddique NA, Khatun N, Rahman MH, Morshed M (2003) Regeneration of shoot from cotyledon derived callus of chickpea (Cicer arietinum L.). Pakistan Journal of Biological Sciences6(15): 1310-1313.
- Kiran SG, Sujata KG, Srinath MR, Kavi PBK (2010) Direct somatic embryogenesis and plant regeneration from immature explants of chickpea. Biologia Plantarum 54(1): 121-125.
- Neelam A, Reddy CS,Reddy GM (1986) Plantlet regeneration from callus cultures of chickpea (Cicerarietinum L). Plant Cell Reports 7: 21-22.
- Kaberi D, Raihan MD, Mahbubur SM, Akhtar SH (2013) Callus induction in chickpea (Cicer arietinum L). Banat Journal of Biotechnology5(7): 92-95.
- Arora A, Chawla HS (2005) Organogenesis plant regeneration via callus induction in chickpea (Cicer arietinumL.)-Role of genotype, growth regulators and explants. Indian Journal of Biotechnology 4: 251-256.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...