Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6062
Case Report(ISSN: 2638-6062) 
Congenital Fibre Type Disproportion: More Than a Congenital Myopathy Volume 4 - Issue 5
Farhat Emna1,2, Miladi Najoua2*, Marrak Mohamed Souheil1 and Chaabouni Myriam3
- 1Neurologist, Tunisia
- 2Child neurologist, Tunisia
- 3Neuropathology lab, Clinique les Jasmins, Tunisia
- 4Genetic lab,Clinique les Jasmins, Tunisia
Received: March 27, 2023; Published: April 03, 2023
*Corresponding author: Miladi Najoua, Child neurologist, Tunis, Tunisia
DOI: 10.32474/PRJFGS.2023.04.000200
Abstract
We report three patients who had shown hypotonia and muscle weakness from early childhood with isolated fibre type disproportion on muscle biopsy (MB), initially diagnosed as having congenital myopathy (CM). The genetic analysis revealed mutations in the Protein-O-Mannose Kinase (POMK) gene in patient 1, the D Myotonin-Protein Kinase (DMPK) gene in patient 2 and the collagen 13A (COL13A) gene in patient 3, confirming respectively the diagnosis of congenital muscular dystrophy (CMD), type 1 myotonic dystrophy (MD) and autosomal recessive congenital myasthenic syndrome (CMS) type 19. Reconsidering the diagnosis allowed us to propose a treatment for one patient, to look for cardiac complications in one patient and to establish genetic counselling for all patient’s family members. We emphasize throw these observations that congenital fibre type disproportion (CFTD) is a nonspecific histological pattern which could be associated to various types of dystrophic and non-dystrophic muscle disorders, and we discuss its place among the spectrum of CM.
Keywords: Congenital myopathy; congenital fibre type disproportion; gene; mutation
Introduction
CFTD is a histological entity characterized by type 1 fibres smaller by at least 12% than type 2 fibres, and absence of other pathological features on MB [1]. Clinical manifestations are nonspecific, there is also no known specific genetic marker or distinct pattern of inheritance [2]. CFTD has been recognized for decades as a subtype of CM. Mutations of the α-tropomyosin slow (TPM3) gene are the most frequently associated with aetiology [3]. In the last years, several reports have subsequently documented cases of CFTD associated with various types of diseases including limb girdle muscular dystrophy (LGMD), CMD, MD and CMS. These findings suggested that CFTD may represent a larger spectrum of disorders [4]. We report phenotype and genotype description of three patients who had shown muscle weakness from early childhood and typical pattern of CFTD on MB allowing to retain initially the diagnosis of CM. The genetic analysis revealed new genes in relation with other types of myopathies.
Patient 1
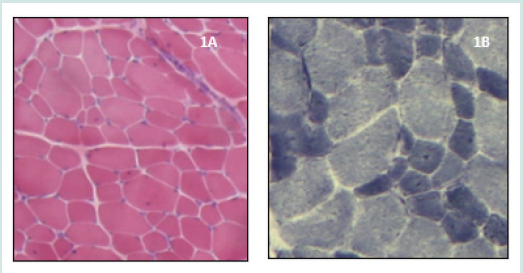
The patient is an 8-year-old girl with a familial history of firstdegree consanguineous parents, 2 similar cases in cousins, severe neonatal hypotonia and respiratory impairment with delayed motor and speech development. At examination she had weakness in the proximal extremities. Serum-creatine kinase (CK) plasma level was elevated, and the electromyogram electromyography (EMG) showed a myopathic pattern. Brain MRI was normal. MB concluded to a CFTD (Figures 1A&1B). The Whole Exome Sequencing (WES) revealed a missense homozygous mutation in the POMK gene, confirming the diagnosis of CMD.
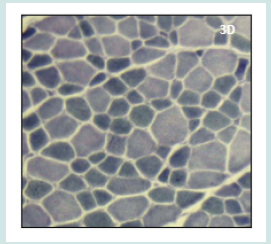
Figure 1: On Hematoxylin and Eosin (H&E) stain (Figure 1A) there are few central nuclei, no necrosis, and no increase of connective tissue. On nicotinamide adenine dinucleotide tetrazolium reductase oxidative enzymatic (NADH) stain (Figure 1B) there is a predominance of type 1 fibres which are significantly smaller than type 2. Figure 1: Histopathological features of patient 1.
Patient 2
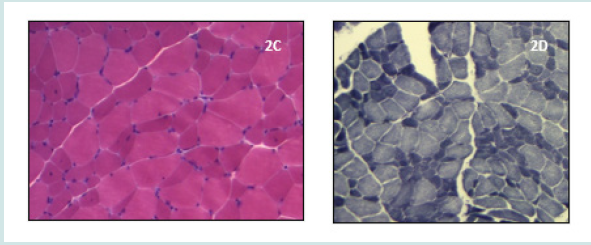
The patient was born of non-complicated twin pregnancy, had delayed speech development, major learning problems and excessive slowness of movement. On the first examination she was12 years-old, showed a slim phenotype, lordotic stance, facial involvement with ptosis, dysphonia, and moderate proximalpredominant limb muscle weakness (Figures 2A&2B). On MB we found non-specific myogenic changes with variation in fibre size without any dystrophic signs, and a selective atrophy of type 1 fibres (Figures 2C&2D). CK levels were slightly increased. EMG revealed myotonic discharges. This sign prompted the analysis of the DMPK gene allowing the diagnosis of type 1 MD, with 61 expanded CTG repeats.
Figure 2: Images of patient 2: A marked atrophy of the temporal, masseter, and sternocleidomastoid muscle (Figure 2A), a slim phenotype with lordotic stance (Figure 2B).

Figure 3: On H&E stain (Figure 2C) there is varied muscle fibre size, few central nuclei, and no increase of connective tissue. On NADH stain (Figure 2D) there is a selective atrophy with predominance of type 1 fibres. Figure 2: Histopathological features of patient 2.
Patient 3
The patient had a history of congenital hypotonia with delayed motor development. He reported a progressive course of muscle weakness. At the age of 14 he underwent scoliosis surgery, and a non-invasive nocturnal ventilation was recently indicated due to a severe sleep apnea. At time of first examination, he was 34 years old, was still able to walk and had severe weakness of facial, axial, and proximal limbs (Figures 3A-3C). EMG was myogenic, CK levels were normal. MB concluded to CFTD (Figure 3D). The WES allowed the identification of a homozygous mutation in the COL13A gene, confirming the diagnosis of the autosomal recessive CMS type 19. He was treated with pyridostigmine and salbutamol with progressive improvement of his state.
Figure 4: Images of patient 3: A severe fatigable weakness of facial muscles (Figure 3A), scoliosis (Figure 3B), proximal limb weakness with lordotic stance (Figure 3C).

Figure 5: On NADH stain (Figure 3D) there is a selective atrophy with predominance of type 1 fibres. Figure 3: Histopathological features of patient 3.
Discussion
The core histologic feature that defines CFTD is the selective atrophy of type 1 fibres (slow twitch), with a mean diameter being at least 12% smaller than the diameter of type 2 fibres (fast twitch) on MB. This disproportion must be the main structural pathological change and the diagnosis is often made after ruling out other histopathological findings especially rods, cores, and central nuclei. CFTD is clinically characterized by hypotonia and mild-to-severe generalized muscle weakness at birth or within the first year of life. Multiple joint contractures, scoliosis, long thin face and high arched palate are classic features. Ptosis, facial muscle weakness, ophthalmoplegia and dysphagia were reported in patients with mutations in TPM3, actin alpha 1 skeletal muscle (ACTA1) and ryanodine receptor type 1 (RYR1) genes [5,6]. Intelligence is usually normal, central nervous system abnormalities have been described in some cases [7]. Respiratory involvement is common, reportedly being seen in almost 30% of patients, however cardiac complications are rare [8]. The CK levels are normal or slightly increased. The EMG pattern is not specific, described as normal, myogenic, or neurogenic in reported cases of CFTD [9] The prognosis is often considered to be benign. In a review of Clarke and North of 64 cases, most patients follow a relatively benign course with limb weakness that improves with age. In the same review, 10% of patients died of severe respiratory failure.
To date, 10 genes have been shown to cause CM with CFTD (Table 1). Autosomal recessive, autosomal dominant and X-linked inheritance patterns are known [10,11]. Mutations in the selenoprotein N1 (SEPN1), ACTA1, RYR1 and TPM3 genes are the most frequent causes. There has been a long debate on whether CFTD is a disease entity or if it is ‘pathology in search of a disease’ [12,13], and this was for several reasons. First, the disproportion of muscle fibres is a feature of almost all subtypes of CM with structural defects, which may constitute a problem of differential diagnosis. In some cases of centronuclear, nemaline or core myopathies, muscle biopsies showed in early stages of the disease an appearance of pure CFTD without other pathological defect. Furthermore, it was demonstrated that histological abnormalities are different in MB taken from the same patient at different ages, or members from different generations of the same family, suggesting that CFTD can be a transient pattern [14-16]. Second, in addition to CM, various neuromuscular disorders in which histological aspect of CFTD is prominent were reported. The use of WES techniques allowed the identification of new genes, broadening the phenotype and genotype spectrum of this entity.
Cases of muscular dystrophy including Ulrich CMD [17], calpain LGMD [18] and laminopathies [19] were reported. For these reasons, CFTD is becoming a more and more nosologically questionable entity. In fact, among all types of CM, only core diseases, nemaline myopathy and centronuclear myopathy are firmly established as distinct entities [20]. In contrary, spheroid body, reducing body, sarcotubular, predominance of type 1 fibres and CFTD myopathies are not well delineated entities, and their place among subtypes of CM remains uncertain There was widespread concern that these entities are too nonspecific to be of clinical use [21,22]. In our patients, the neonatal onset of symptoms, myogenic pattern on EMG and isolated pattern of CFTD on MB led us to retain the initial diagnosis of CM. The clinical phenotypes were not homogenous, but similar to the features of CM with CFTD. Patient 1 high CK levels (more than five times the upper normal value) and the progressive evolution of patient 3 were atypical for a CM. Results of the genetic analysis excluded the diagnosis of CM and allowed patients to be reclassified to other diagnosis. To our knowledge mutations of POMK1 and COL13A genes were never reported with histological pattern of CFTD. CMD can show CFTD pathology.
In his case series of 10 MB from 8 patients with genetically proven Ulrich CMD, Schessel, et al. highlights the presence of pattern of CFTD and absence of dystrophic changes especially in early stages of the disease. CFTD can also be seen in a MB from patients with congenital MD. Tominaga et al. reported 28 unrelated patients who were pathologically diagnosed as CFTD, 14% of them had marked expansion of trinucleotide (CTG) in the DMPK gene [23]. Histological findings of CTFD were never reported with juvenile onset of congenital MD to our knowledge. Finally, type 1 fibre predominance and disproportion have been rarely noted cases with CMS, with a single genetically analyzed family having mutation of the DPAGT1 gene (UDP-N-acetylglucosaminedolichyl- phosphate N acetylglucosaminephosphotransferase 1) [24,25]. In our last patient, the second MB, done 30 years after the first, revealed a typical pattern of CFTD. Searching for precise genetic diagnosis allowed us to propose a treatment for patient 3 (diagnosed with autosomal recessive CMS type 19) who received until the age of 34 only supportive treatment. It also motivates us to look for specific cardiac complications in patient 2 (diagnosed with a juvenile form of MD type1) and to establish genetic counselling for his family members.
Conclusion
CFTD remains a controversial entity more and more documented as a histological pattern across various subtypes of myopathies. In our opinion, and after studying the three reported cases, we think that it is better to consider CFTD as a syndrome rather than a formal diagnosis. As the WES analysis becomes more available and the identification of the genetic basis of patients with CFTD is becoming significantly easier, this fact should be motivating for the clinician to look for the precise diagnosis beyond the spectrum of CM. We insist through these observations that we must keep in mind that CFTD can reveal potential treatable or lifethreatening diseases.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this publication.
References
- Clarke NF, North KN (2003) Congenital fiber type disproportion-30 years on. J Neuropathol Exp Neurol 62(10): 977-989.
- Clarke NF, B Ilkovski, S Cooper, Valova VA, Robison PJ, et al. (2007) The pathogenesis of ACTA1-related congenital fiber type disproportion. Ann Neurol 61(6): 552-561.
- Claeys K (2020) Congenital myopathies: An update. Dev Med Child Neurol 62(3): 297-302.
- Imoto C, Nonaka I (2001) The significance of type 1 fiber atrophy (hypotrophy) in childhood neuromuscular disorders. Brain Dev 23(5): 298-302.
- Laing NG, Clarke NF, Dye DE, Liyanage K, Walker KR, et al. (2004) Actin mutations are one cause of congenital fibre type disproportion. Ann Neurol 56(5): 689-694.
- Clarke NF, L Smith R, Bahlo M, North KN (2005) A Novel X-Linked Form of Congenital Fiber-Type Disproportion. Ann Neurol 58(5): 767-772.
- Vestergaard H, Klein HH, Hansen T, Muller J, Skovby, et al. (1995) Severe insulin-resistant diabetes mellitus in patients with congenital muscle fiber type disproportion myopathy. J Clin Invest 95(4): 1925-1932.
- Banwell BL, Becker LE, Jay V, Taylor GP, Vajsar J (1999) Cardiac manifestations of congenital fiber-type disproportion myopathy. J Child Neurol 14(2): 83-87.
- Cavanagh NP, Lake BD, Mc Meniman P (1979) Congenital fibre disproportion myopathy. A histological diagnosis with an uncertain clinical outlook. Arch Dis Child 54(10): 735-743.
- Jaffe M, Shapira J, Borochowitz Z (1988) Familial congenital fiber type disproportion (CFTD) with an autosomal recessive inheritance. Clin Genet 33(1): 33-37.
- Kim WK, Choi BO, Cheon HY, Sunwoo IN, Kim TS (2000) Muscle fiber type disproportion with an autosomal dominant inheritance. Yonsei Med J 41(2): 281-284.
- Dubowitz V (1995) Congenital fibre type disproportion: A pathology in search of a disease, in Dubowitz V (ed): Muscle Disorders of Childhood, 2nd edition, Baillière Tindall, London, UK.
- Clarke NF (2011) Congenital fibre type disproportion-a syndrome at the crossroads of the congenital myopathies. Neuromuscul Disord 21(4): 252-253.
- S Jongpiputvanich, PJ Walsh, BA Kakulas (1995) Minicores and congential fibre type disproportion observed in a family. J Paediatr Child Health 31(3): 253-257.
- Camacho A, Villarejo A, Simón R, Mateos F, Cabello A (2005) Clinical and histologic changes in the follow-up of a congenital myopathy. Pediatr Neurol 33(2): 139-141.
- Bartholomeus MG, Gabreëls FJ, Ter Laak HJ, van Engelen BG (2000) Congenital fibre type disproportion a time-locked diagnosis: a clinical and morphological follow-up study. Clin Neurol Neurosurg 102(2): 97-101.
- Schessl J, Goemans NM, Magold A, Zou Y, Hu Y, et al. (2008) Predominant fiber atrophy and fiber type disproportion in early ullrich disease. Muscle Nerve 38(3): 1184-1191.
- G Vattemi, P Tonin, M Neri, M Marini, F Gualandi, et al. (2009) Calpain 3 deficiency presenting as fibre type disproportion. Neuropathol Appl Neurobiol 35(6): 614-617.
- Sachiko Kajino, Kayo Ishihara, Kanako Goto, Keiko Ishigaki, Satoru Noguchi, et al. (2014) Congenital fiber type disproportion myopathy caused by LMNA mutations. J Neurol Sci 340(1-2): 94-98.
- Ravenscroft G, Laing GN, Bonnemann GC (2015) Pathophysiological concepts in the congenital myopathies: blurring the boundaries, sharpening the focus. Brain 138(Pt 2): 246-268.
- Sang-Jun N, Woo-Kyung K, Tai-Seung K, Seong-Woong K, Eun-Young L, et al. (2006) Fiber type disproportion myopathy and congenital myopathy with type 1 fiber predominance. Yonsei Medical Journal 7(4): 513-518.
- Clarke NF (2011) Congenital Fiber-Type Disproportion. Semin Pediatr Neurol 18(4): 264-271.
- Tominaga K, Hayashi YK, Goto K, Minami N, Noguchi S, et al. (2010) Congenital myotonic dystrophy can show congenital fiber type disproportion pathology. Acta Neuropathol 119(4): 481-486.
- Gurnett CA, Bodnar JA, Neil J, Conolly AM (2004) Congenital myasthenic syndrome: presentation, electrodiagnosis, and muscle biopsy. J Child Neurol 19(3): 175-182.
- Duygu Selcen, Xin-Ming Shen, Joan Brengman, Ying Li, Anthony A. Stans, et al. (2014) DPAGT1 myasthenia and myopathy Genetic, phenotypic, and expression studies. Neurology 82(20): 1822-1830.
- NF Clarke, H Kolski, DE Dye, Lim E, Smith RLL, et al. (2008) Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol 63(3): 329-337.
- NF Clarke, LB Waddell, ST Cooper, Perry M, Smith RLL, et al. (2010) Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Hum Mutat 31(7): E1544-E1550.
- Brandis A, Aronica E, Goebel HH (2008) TPM2 mutation. Neuromuscul Disord 18(12): 1005.
- NF Clarke, W Kidson, S Quijano-Roy, Estournet B, Ferreiro A, et al. (2006) SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol 59(3): 546-552.
- Mercier S, Lornage X, Malfatti E, Marcorelles P, Letournel F, et al. (2017) Expanding the spectrum of congenital myopathy linked to recessive mutations in SCN4A. Neurol 88(4): 414-416.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...