Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4595
Short Communication(ISSN: 2637-4595) 
Witt and the Theory of Dyeing Volume 3 - Issue 4
Fathi Habashi*
- Laval University, Canada
Received: September 27, 2019; Published: October 04, 2019
*Corresponding author: Fathi Habashi, Laval University, Quebec City, Canada
DOI: 10.32474/LTTFD.2019.03.000167
Abstract
Otto N. Witt (1853-1915) was the first to put a theory of dyeing in 1876 based on chromophore and auxochrome. He also conceived vacuum filtration as a method for rapid filtration.
Working in England
Otto Nicolaus Witt (1853-1915) was born in Saint Petersburg, Russia, the son of a German pharmacy professor. He was in school when the family moved again to Switzerland. He studied chemical technology at the Federal Polytechnic School in Zurich finishing his degree in 1873. He found a job in a steel works in Duisburg, Germany but within months was back in Zurich making colors for cotton printers. He then joined Williams, Thomas & Dower in west London.
Back to Germany
Developments in the dyeing industry were so interesting that Witt went back to university in Germany to work in Victor Meyer’s lab. His thesis in 1875 reported on the reactions of aromatic nitrosamines and their reactions with dichlorobenzene.
Vacuum Filtration
Witt suggested placing a disc riddled with holes into a funnel, with filter paper laid on top, moistened to create a seal. He wrote a paper in 1886 describing an improved method for vacuum filtration. Witt’s system worked, but it required care to use because the plates leaked round the sides and could slip sideways. Almost immediately the method was improved by Ernst Büchner (1850- 1924) who developed his now funnel in which the plate is integrated into the funnel itself.
Dyeing at the Time of Witt
In 1840 when Queen Victoria married her cousin Prince Albert who was prince of Saxe-Coburg-Gotha, a region near Justus von Liebig’s laboratory, the prince who was active in promoting scientific education in England, was able to found a College of Chemistry in London with August Hofmann (1818-1892) as its director. Hofmann had worked with Liebig on separating the components of coal tar before being invited to England. The discovery of mauveine in 1853 by William Perkin (1838-1907) in Hofmann Laboratory in London was soon followed by the discovery of other brightly colored compounds obtained by the oxidation of mixtures of anilines. The new colors began to revolutionize the textile industry. In 1858, the graduate student Peter Griess (1829-1888), working in Hermann Kolbe’s lab at the University of Marburg, discovered the diazotization reaction when he treated anilines with nitrous acid. Griess’s discovery opened up the possibility of coupling together a range of aromatic molecules that were being discovered across Europe.
Griess joined Hofmann’s group in London, where he continued to study his coupling reaction. Around the same time, Heinrich Caro (1834-1910) working with his employer John Dale in Manchester, developed the first two azo dyes: Manchester Yellow and Bismarck Brown. Caro had already returned to Germany to join Badische Anilin- und Soda Fabrik in Ludwigshafen in Germany known as BASF. Carl Graebe (1841-1927) and Carl Liebermann (1842-1914) working for BASF synthesized the ancient dye alizarin in 1868. Adolph von Baeyer (1835-1917) Professor of chemistry in Munich researched the synthesis of the ancient dye indigo and determined its structure in 1870. The same year he prepared indigo. The result was that German firms like BASF, Hoechst, and Baeyer or the German-Belgian firm Aktiengesellschaft für Anilinfabrikation known as AGFA, steadily undercut the British in both price and quality. The British dye industry faded.
Theory of Dyeing
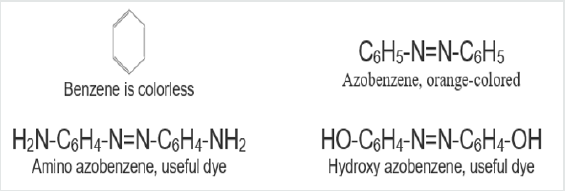
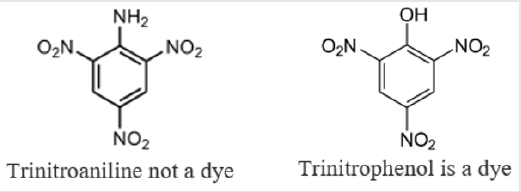
In a paper published in 1876, he speculated that colored compounds were the result of a grouping of atoms – he called a chromophore. By adding the auxochrome, the dye could be made to stick to a fabric. According to Witt, benzene is colorless but azobenzene is orange colored. Although it is strongly colored it is devoid of dyeing power. Groups such as azo group, -N=N-, are called chromogenes. In order to convert them into useful dyes the introduction of an auxochromes, i.e., a salt-forming groups is necessary. Thus, while azobenzene is not a dye both aminoazobenzene and hydroxy- azobenzene are useful dyes. The presence of both chromophore and auxochrome does not necessarily indicate that the substance is a useful dye. For example, trinitroaniline contains both the chromophre group, NO2, and the salt forming auxochrome group, NH2, but it is not a useful dye because the basic character of the auxochrome is neutralized by the strongly acidic character of the chromophore. On the other hand, trinitrophenol (picric acid) is a useful yellow dye.
Conclusion
Witt’s theory was later modified when it was discovered that the chromophore is usually electron-withdrawing, and auxochromes are normally electron-donating. The two groups are connected by a conjugated system.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...