Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4595
Mini Review(ISSN: 2637-4595) 
Study on Surface Morphology and Chemical Transformation of Uniform Fabric Treated with A Silane-Based Quaternary Ammonium Compound Volume 4 - Issue 5
Kushal Desai, Bharat Patel* and Dharmesh Solanky
- Department of Textile Chemistry, Faculty of Technology and Engineering, Kalabhavan, The Maharaja Sayajirao University of Baroda, India
Received:March 01, 2022 Published: March 15, 2022
*Corresponding author: Bharat Patel, Department of Textile Chemistry, Faculty of Technology and Engineering, Kalabhavan, The Maharaja Sayajirao University of Baroda, India
DOI: 10.32474/LTTFD.2022.04.000197
Abstract
In this research, an attempt has been made to investigate the effects of a quaternary ammonium compound (QAC) namely 2-Hydroxyethoxydimethoxysilyl on Cotton, Polyester/Viscose, and Polyester/Cotton blend fabrics. The treatment decreased the whiteness and brightness by increasing yellowness in the uniform fabrics. The SEM image indicates that the treatment with QAC makes the fabric surface smoother compared to its counterpart. The EDX results further confirm the existence of QAC in the modified textile fabrics. Further, the presence of various groups incorporated in the fabric due to the treatment is confirmed by the FTIR spectrum of treated fabrics.
Keywords: Chemical composition; Quaternary ammonium compound; Uniform fabrics; Surface morphology; Structural property
Introduction
Research nowadays on textile fibers has been increasingly concentrated on developing modified fibers having better properties than the original fibers. This has paved the way for numerous possibilities of modifying cotton fiber, to overcome the deficiencies present in the fiber. Several changes can be made in the physical and chemical properties of modified cotton fiber, which generally are done by modification of the fiber. Chemical modification of textiles is the most common and convenient process to improve the functional properties of textiles. In this perspective, the cationization of cotton is the most effective treatment that may help to solve the environmental problems associated with the dyeing of cotton. Generally, cotton is cationized with quaternary ammonium compounds to improve its dyeability with direct and reactive dyes [1,2]. Quaternary ammonium compounds, biguanides, amines, and glycoprotein show polycationic, porous, and absorbent properties. Fibers finished with these substances bind microorganisms to their cell membrane and disrupt the lipopolysaccharide structure resulting in the breakdown of the cell. Based on poly (hexamethylene) biguanide hydrochloride (PHMB) is claimed to possess low mammalian toxicity and a broad spectrum of antimicrobial activity. PHMB is particularly suitable for cotton and cellulosic textiles and can be applied to blends of cotton with polyester and nylon.
Generally, it is used in swimming pools, cosmetics, contact lens solutions, and natural textiles. Another product reported in the literature, based on octadecylaminodimethyltrimethoxysilylpropylammoniumchloride can be applied by either exhaust or continuous methods. After application, a curing step is required to form a siloxane polymer coating on the fiber surface. This coating immobilizes the antimicrobial part of the molecule (the quaternary nitrogen) and provides the necessary durability to laundering [3,4]. The cationization treatment for uniform textile materials is necessary to enhance its functional properties and to safeguard the textile products from staining, discoloration, and quality deterioration. It is important to take into account the impact of the cationization treatment on the surface morphology and the chemical transformation of the finished substrates. In the present investigation, an attempt has been made to evaluate the effect of a QAC; silane-based quaternary ammonium compound (zycrobial) treatment on the surface morphology of Polyester/Viscose (P/V), Polyester/Cotton (P/C) blend, and 100% Cotton (C) fabric by SEM technique and the modification in the chemical structure due to the treatment was analyzed by FTIR spectroscopic technique.
Materials
Fabrics
The three types of fabrics were selected for uniform fabrics namely, Polyester/Viscose (P/V), Polyester/Cotton (P/C) blend, and 100% Cotton (C). To remove the finish and other hydrophobic impurities from all the three selected fabrics. The fabrics were treated with a bath containing 5 gpl non-ionic detergent and 2 gpl sodium carbonate for 30 minutes at 800C temperature. The fabrics were then washed thoroughly in running water, neutralized, washed again in running water, and finally dried under shade. The pretreatment process was carried out in the L.G. Direct Drive washing machine. The pH of the fabrics was checked to neutral before further processing.
Chemicals
The QAC; silane-based quaternary ammonium compound (zycrobial) material supplied by Zydex industries limited, Vadodara. Other chemicals i.e., acetic acid, R-77,and sodium carbonate used in this experiment were of analytical grade and used without further purification.
Experimental methods
The experimental plan was based on the application of QAC on P/V, P/C, and C fabrics by pad–dry- cure method.
QAC treatment of uniform fabrics
Treatment of QAC on fabric was done by padding technique. In pad application, the fabric immersed in liquor contain the required amount of QAC agent (zycrobial- 30 gpl and 50 gpl) and passes through the padding mangle at 2.5 kg/cm2 pressure using laboratory two bowl padding mangle. The fabric was subsequently dried and cured at room temperature.
Testing and Analysis
The treated fabric in terms of change in their morphological, and chemical properties was evaluated by SEM, EDX, and FTIR spectroscopy analysis.
Whiteness, Yellowness and Brightness Index
The spectrophotometer (Spectrascan-5100) was used to measure the whiteness, yellowness, and brightness index of fabric samples. The whiteness and yellowness index were measured as per the ASTM E313 method, and the brightness index was measured according to TAPPI 452/ISO 2470 method. All the measurements were done at 20 observers in % reflection and large diameter mode.
Surface characteristic by Scanning Electron Microscopy (SEM) Analysis
The surface of treated fabrics was imaged through Scanning Electron Microscope (SEM), (model JSM5610LV, version 1.0. Jeol, Japan). The small fabric sample adhered to a carbon-coated aluminum sheet further attached to a circular sample holder was observed under vacuum using the scanning electron microscope. Scanned images with different magnification and resolution were recorded on the computer.
Energy Dispersive X-ray Spectrometry (EDX)
The elements in the polymer structure were detected and measured using an energy dispersive X-ray analysis technique. The elemental analysis of the treated and untreated samples was performed on a scanning electron microscope (SEM) using Oxford Inca Software.
FT-IR (Fourier transform infrared spectroscopy)
The chemical composition of the QAC treated and untreated fabrics were recorded using FT-IR Spectroscopy Nicolet is10 FT-IR Spectrometer (Thermo Scientific).
Results and Discussion
In this section, the morphological and chemical transformations due to the QAC treatment were analyzed by a computer color matching system (CCM) in terms of whiteness, brightness, and yellowness index, and the surface morphology of treated fabric was observed by SEM. The fabric was further characterized by EDX and FTIR for their chemical composition.
Effect on Whiteness, Yellowness and Brightness Index
The results in terms of whiteness, yellowness, and brightness index are shown in Table 1. Results show the QAC treatment decreased the whiteness and brightness index of the P/V fabric with a minor increase in yellowness of the sample. From Table 1 and corresponding Figure 1, it can also be seen that the treatment increases the yellowness of the sample, makes the sample slide duller by decreasing whiteness and brightness index. This effect was found more pronounced in the case of P/C and cotton fabric samples.
Effect on Surface morphology of uniform fabrics
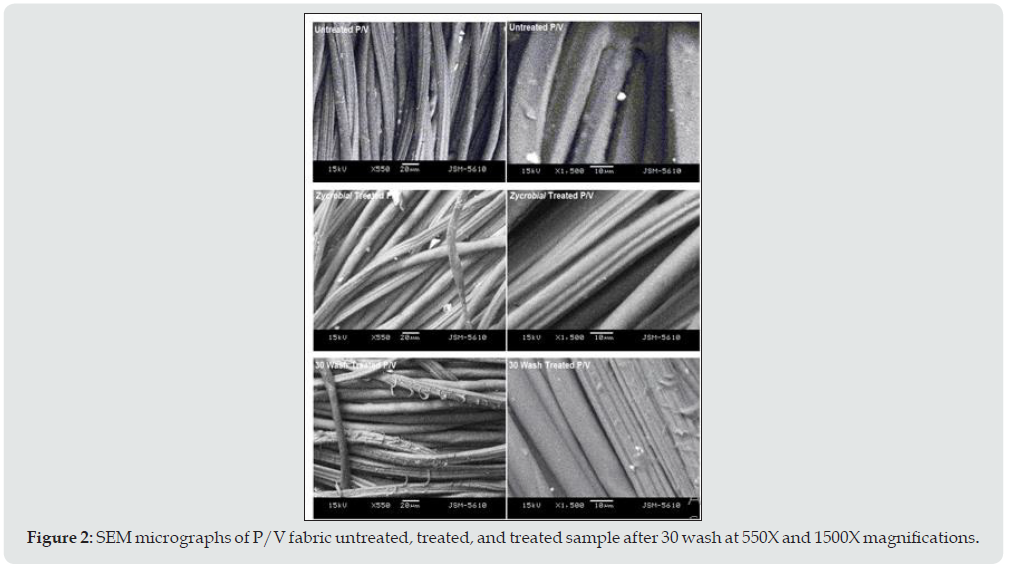
Figure 2 shows microphotographs of untreated, treated, and treated P/V fabric samples after 30 wash. From the surface morphology of the untreated sample at X550 and X1500 magnification, a clear surface of filaments with some residual particles of the finished product was observed. From the microphotographs of the QAC treated sample, the surface is seen clear and smooth compared to the SEM of the untreated sample. The treatment provides a uniform coating on the surface of the fibers due to its cationic nature and having 18 carbon atoms in its chain [5]. However, samples after 30 wash some fibrillation were observed which may be due to the rupture of viscose filament after 30 wash treatment.
Figure 2: SEM micrographs of P/V fabric untreated, treated, and treated sample after 30 wash at 550X and 1500X magnifications.
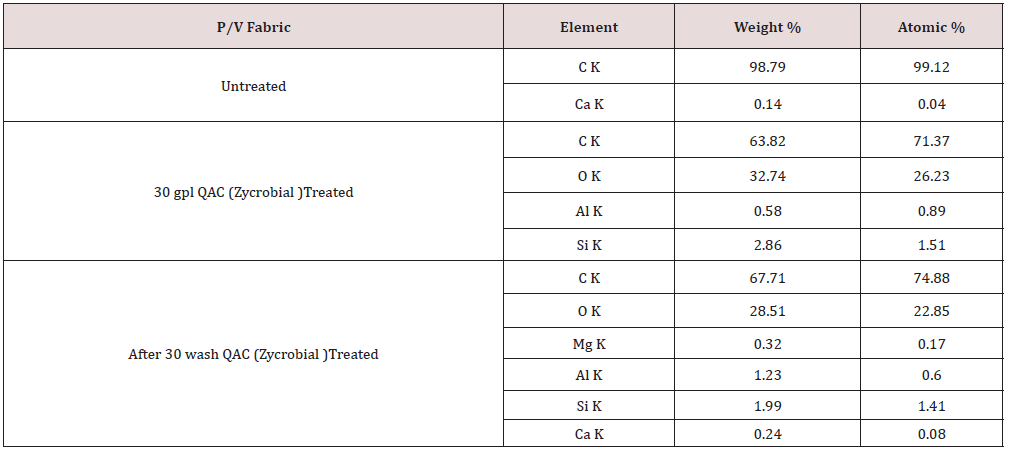
Elemental Analysis by Energy Dispersive X-ray Spectrometry (EDX)
The elemental analysis of P/V samples with and without QAC treatment was recorded using Oxford-Inca software on a scanning electron microscope. From Figure 3 various peaks for carbon, calcium, iron, etc. were observed in the case of untreated P/V fabric. Fabric sample with the treatment and treated sample after 30 wash show peaks for Si as element indicates the presence of silane in treated sample. The present higher quantity of oxygen in the treated sample as seen from Table 2 indicates that the silane is in the form of oxide or dioxide. The presence of a peak for Si in the treated sample indicates that the silane remains in the fabric even after 30 wash. Various peaks for carbon, Ca, Al, etc. have also appeared in Figure 3. This may be due to the use of carbon-coated aluminum sheets as a sample holder.
Analysis of chemical transformation by FT-IR
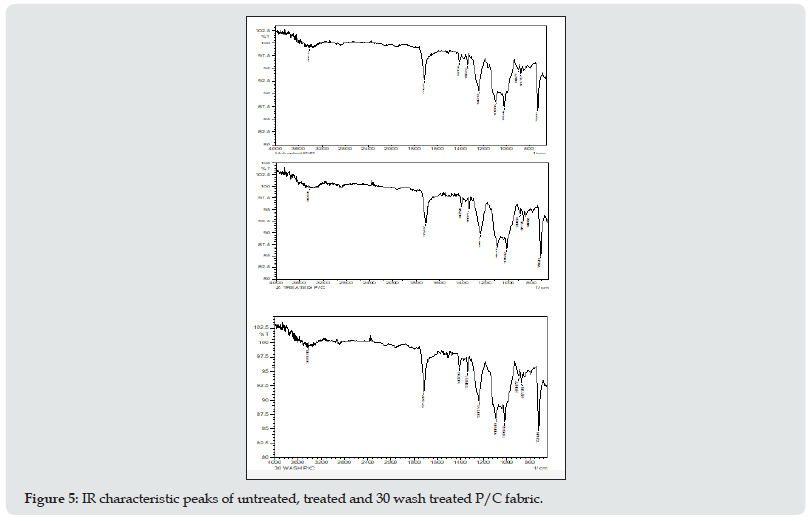
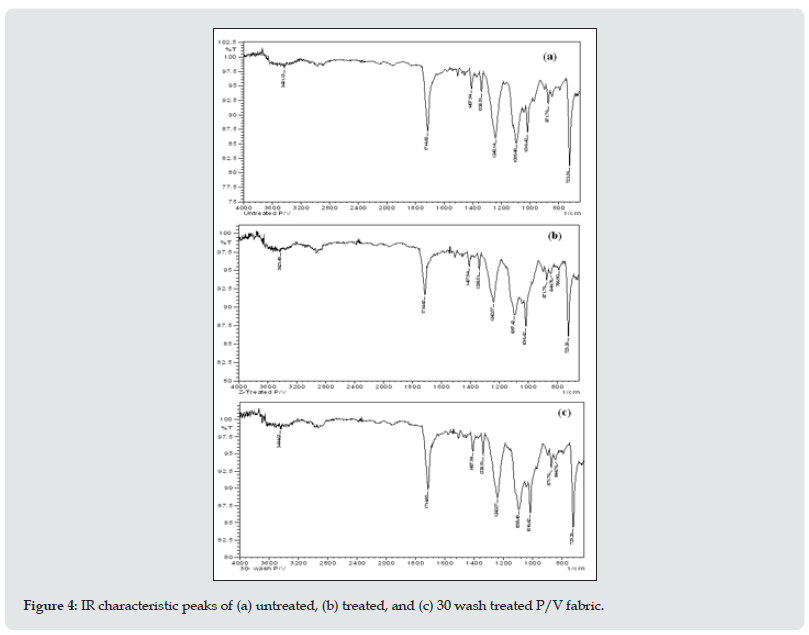
The FT-IR spectra and absorption peaks for P/V fabric with and without antimicrobial treatment along with spectra of the treated samples after 30 wash are shown in Figure 4. From the results, the presence of silane bonded with –OH group of fabric by Si-O-Si continuous linkage was characterized at around 800 &1086 cm-1, which are attributed to Si-O bending vibration band and Si-O-Si antisymmetric stretching vibration band respectively. The peaks in the FT-IR spectra of p/v blend fibers appeared in the range of 600-4000 cm-1 (Figure4). The waves were assigned as follows: 1714 cm-1 (C=O), 1407 cm-1 (aromatic ring), 1338 and 1016 (carboxylic ester or anhydride), 1095 cm-1 and 1016 cm-1 (O=C–O–C or secondary alcohol), 971 cm-1 (C=C), 871 cm-1 (five substituted H in benzene), 844 cm-1 (two neighboring H in benzene), 723 cm-1 (heterocyclic aromatic ring). The main structure of the polyester sample had ester, alcohol, anhydride, aromatic ring, and heterocyclic aromatic rings. Alcohol was able to react with anhydride and produce ester groups. That was the reason there was still alcohol and anhydride as residual reactants left in the polyester. The carboxyl, ester, anhydride, and alcohol groups showed the polyester fabric was not pure PET. The peak at 1407 cm-1 corresponded to the aromatic ring which was a stable group. It was the characteristic absorption peak of PET. Polyester/cotton blended fabric used for the experiments contains polyester (67%) as a major component. Therefore, the peaks appearing in the 723 cm-1 can be attributed to the polyester component of the blend. The peak at 1712 cm-1 can be assigned to the stretching vibration of the C=O group in ester. The peaks at 1240 cm-1 and 723 cm-1 can be assigned to asymmetric C=O stretching vibration and aromatic C-H out-of-plane vibrations respectively. A weak peak observed around 896 cm-1 is attributed to the stretching of the C-N (+) bond formed in the treated fabric.
Figure 4: IR characteristic peaks of (a) untreated, (b) treated, and (c) 30 wash treated P/V fabric.
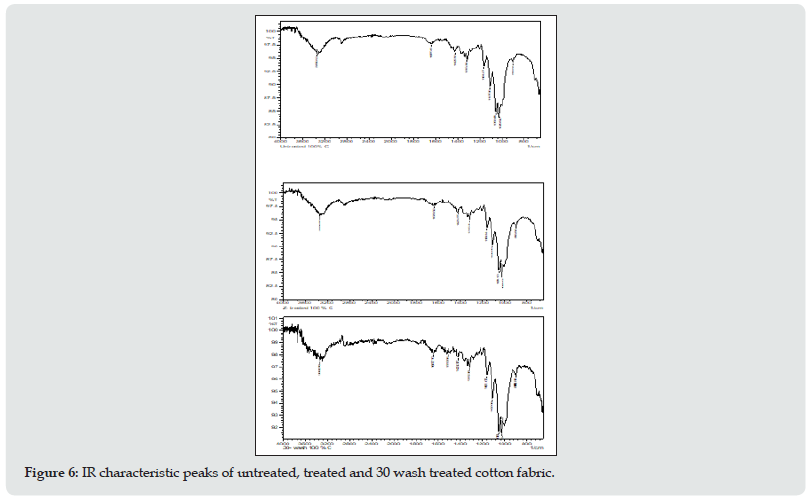
The intramolecular changes of the cotton fabrics before and after QAC treatment also after 30 wash are characterized by FT-IR spectral analysis, shown in Figures 6 The absorption in the region of 3600-3100 cm-1 was due to the stretching of –OH groups [6-8] and at 3000-2800 cm-1 to the CH stretching, the slight increase of these contents after the treatment may indicate that hydrogen bonds and methyl and methylene of cellulose had some additional attachment [8-11]. The band at 1635 cm-1 arose from the H–O–H bending of the absorbed water. The symmetric –C–H bending occurred at 1423 cm-1. The band at 1372 cm-1 was assigned to –OH bending and at 1315 cm-1 to C–C and C–O skeletal vibrations. While the bands at 1051 and 1029 cm-1 indicated C–O stretching at C3, C–O stretches at C6 and C–C stretching [12-15]. These C–O stretching bands gave slight shoulders at 1161 cm-1 which represented the antisymmetric bridge stretching of C–O–C groups in cellulose. The band at 1107 cm-1 corresponded to asymmetric glucose ring stretching. After the treatment, the bands at 1161 cm-1 decreased slightly and 1107 cm-1 increased slightly.
Conclusions
QAC; a silane-based quaternary ammonium compound (zycrobial) namely 2-Hydroxyethoxydimethoxysilyl, can be applied on P/V, P/C, and C fabrics by economical pad-batch technique. The treatment decreased the whiteness and brightness by increasing yellowness in the fabric. The SEM image indicates that the treatment with QAC makes the fabric surface smoother than the untreated surface of the same fabric. The EDX results further confirm the existence of QAC in the treated textile fabrics. The presence of various groups incorporated in the fabric due to the QAC treatment is confirmed by the FTIR spectrum of treated fabrics.
Acknowledgment
The authors are thankful to Mr. J Sridhar (Vice President- Textile), Mr. P Pandey, and Ms. S Vijay of Zydex Industries for extending their support to complete this research work.
References
- BH Patel, RD Pachauri, KU Desai (2014) Changes in cotton treated with choline chloride, The Indian Textile Journal, 124(10): 74-78.
- BH Patel, RDPachauri KU Desai (2014) Effect of salt in the dyeing of choline chloride pretreated cotton with reactive dyes, Cotton Bangladesh 14(2): 22-24.
- WD Schindler, PJ Hauser (2004) Chemical finishing of textiles, chapter-15, p.165.
- BH Patel (2005) Improved dyeability of cotton by chemical modification. The Indian Textile Journal 115(1): 41-46.
- Farzaneh J, Ali Ashjaran (2012) The antimicrobial effects of Zycrobial on cotton and cotton/polyester blend fabrics in the presence of the different dyes and treatments. World applied sciences journal 19(1): 63.
- Y Marechal, H Chanzy (2000) The hydrogen bond network in Ibeta cellulose as observed by infrared spectrometry. J Mol Struct 523: 183.
- J Blackwell, P Vasko, JL Koenig (1970) Infrared and Raman spectra of the cellulose from the cell wall of Valoniaventricosa. J Appl Phys 41: 4375.
- J Sugiyama, J Persson, H Chanzy (1991) Combined infrared and electron diffraction study of the polymorphism of native celluloses, Macromolecules 24: 2461.
- A Michell (1990) Second derivative FTIR spectra of native celluloses. Carbohydr Res 197: 53.
- A Michell (1993) Second-derivative FTIR spectra of native celluloses from Valonia and tunicin. Carbohydr Res 241: 47.
- S Kokot, B Czarnik-Matusewicz, Y Ozaki (2002) Two-dimensional correlation spectroscopy and principal component analysis studies of temperature-dependent IR spectra of cotton-cellulose, Biopolymers 67: 456.
- A Watanabe, S Morita, S Kokot, M Matsubara, K Fukai, et al. (2006) Drying process of microcrystalline cellulose studied by attenuated total reflection IR spectroscopy with two-dimensional correlation spectroscopy and principal component analysis. J Mol Struct 799: 102.
- H Kaczmarek, D Oldak, P Malanowski, H Chaberska (2005) Effect of short wavelength UV-irradiation on ageing of polypropylene/cellulose compositions. Polym Degrad Stab 88: 189.
- Y He, Y Pang, Y Liu, X Li, K Wang (2008) Physicochemical Characterization of Rice Straw Pretreated with Sodium Hydroxide in the Solid State for Enhancing Biogas Production, Energy Fuels 22: 2775.
- J Li, L Zhang, F Peng, J Bian, T Yuan, et al. (2009) Microwave-assisted solvent-free acetylation of cellulose with acetic anhydride in the presence of iodine as a catalyst. Molecules 14: 3551.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...