Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4595
Review Article(ISSN: 2637-4595) 
Polyacralamide – A Potential Option of Salt and Alkali Free Reactive Dyeing on Cotton Volume 5 - Issue 2
Ashraful Alam1, Neaz Morshed2, Zakaria Ahmed1*, Taslim Rahman3 and Pulak Talukder1
- 1Mechanical Processing Division, Bangladesh Jute Research Institute, Bangladesh
- 2Department of Yarn and Fabrics Production, Bangladesh Jute Research Institute, Bangladesh
- 3Department of Microbiology, Bangladesh Jute Research Institute, Bangladesh
Received:August 25, 2022 Published: September 09, 2022
*Corresponding author: Zakaria Ahmed, Mechanical Processing Division, Bangladesh Jute Research Institute, Bangladesh
DOI: 10.32474/LTTFD.2022.05.000207
Abstract
In present experiment, the fibre modification technique based on polyacrylamide was used where the effects of the characteristics of the cationic agent and the pretreatment conditions on dye ability of reactive dye were investigated. It has been observed that the fixation and K/S values of the reactive dyes on the cationic cotton were improved compared with those on the untreated one in the presence of salt and alkali. The maximum K/S value (19.673) found with 4% polyacrylamide and normal process K/S value (13.135). The use of polyacrylamide influences the treatment of K/S value wash and rub fastness of the salt-free dyeing were satisfactory; and anti-crease property, tensile, tear strength and handling of the cationic cotton were also good compared with the of the normal dyeing process.
Keywords: Reactive Dye; Polyacralamide; Fibre
Introduction
The textile colouration and finishing industry is one of the major contributors to environmental pollution [1,2]. This is mainly due to the discharge of nonbiodegradable inorganic salts, alkalis, other processing aids and organic matter such as dyes to the effluent. The effluent treatment can play a significant role in reducing discharge pollution. However, these treatments are expensive and produce highly concentrated solid wastes [3]. Therefore, the better approach would be to improve the textile processing technologies and chemistry for reducing the discharge pollution. The reactive dyes are known as the best for cotton as they show wide range of colours, ease of application and better fastness properties. However, all the reactive dyeing systems require huge amounts of electrolyte to exhaust and alkali to fix the dye. Reactive dyes are colorants used mainly on cotton to achieve high wash fastness on leisurewear. The basis for their good wash fastness is the formation of a covalent bond to cellulose chains during the fixation step. Unfortunately, fi ber fixation is always accompanied by alkali-induced dye hydrolysis, leading to dye molecules that cannot undergo fixation with cel lulose. Bi functional reactive dyes containing monochlorotriazine and sulphatoethylsulphonic groups give excellent color values, solubility, substainity and diffusion having physical and chemical properties of dye fabric, were controlled by the chemical structure of dye and dyeing conditions. Bi functional dyes were found more stable when compared with mono functional reactive dyes [4].
Reactive dyes, the most majestic dyes, lead the cotton dyeing industries still today as they exhibit versatile amenities such as full ranges of shade, convenient fastness properties and ease of application. Implications of these dyes demand a great deal of electrolyte to subdue the electric charge repulsion between dye and fiber. But use of high amount of electrolyte and unfixed dye contributes to environment pollution among which salt cannot be exhausted or destroyed. The usage of salt can be eliminated by either the use of cationized reactive dye or modification of cotton. Notable achievements are found for modification of cotton by treating with chitosan, cationic starch, cationic monomer, polymer and dendrimer. Recently attention has been given on preparation of cationic reactive dye to avoid using modifying agent and attained outcomes met the current market demand [5]. To eliminate salt-usage different approaches have been adopted such as cationization of cotton with cationic agent and infliction of cationic reactive dye [5,6]. To incorporate cationic sites by reaction with hydroxyl groups of cellulose through esterification, etherification, grafting or crosslinking reaction is termed as cationization of cotton [7]. Basically quaternary or tertiary amino groups are embodied with cellulose to provide nucleophilic group to cellulose which show greater attraction for anionic dye resulting in formation of ionic bond without salt even alkali [7,8].
These electrolytes are neither exhausted nor destroyed and hence remain in the dye bath after dyeing. Only 60-65% dye utilization is attainable even with the use of salt in the normal dyeing systems. When alkalinity is introduced in the bath to facilitate the formation of covalent bond between the fibre and the functional group of reactive dye, the abundance of hydroxyl ions causes significant hydrolysis of reactive dyes. These hydrolysed dyes are called ‘dead’ dyes as they have no affinity towards cotton and hence remain in the dye bath. Their deposition on the fibre significantly lowers the fastness properties that causes severe wash-offs. Reactive dyeing thus pollutes the environment due to the highly coloured dye bath discharge, and the discharge of high electrolyte concentration. High electrolyte concentration in the effluents causes worst effects due to the evolution of hydrogen sulphide gas, upsetting the balance in biochemistry of aquatic organisms, deposition of alumino-sulphato complex in the concrete pipes, etc. In typical method of dyeing of cotton with reactive dyes, alkali pH should maintain in the dye bath. When the fabric is treated with polyacrylamide (chitosan), the primary hydroxyl groups of cellulose is (partially) modified into amide groups, which intern leads the cellulose to act like as wool fibre and hence reactive dyes can be dyed on cotton at neutral pH in the absence of electrolyte and alkali. Tertiary amine cationic polyacrylamide with high cationization degree was used as a new cationic agent to pretreat cotton with dip-pad-bake method. The obtained cationic cotton was dyed with reactive dyes in the absence of electrolyte and alkali.
This method requires more electrolytes for exhaustion and alkali for fixation. The salt can be reduced by molecular modification of fibre to have higher affinity and attraction towards anionic dyes which results in reduction of colourant, chemical oxygen demand (COD), biological oxygen demand (BOD), total dissolved solids (TDS) and highly toxic chlorinated organic byproduct (AOX) in the effluent. This also reduces no. of wash-offs, eliminates neutralizing treatment, reduces effluent volume, increases productivity due to reduced dyeing time, increases dye utilization and reduces cost of dyeing and effluent treatment. This shows the possibility of dyeing of cotton in neutral pH. Polyvinylamine Chloride (PVAmHCl) has been used as a physical modifying agent. Due to its wide range of properties, PVAmHCl has found use in catalysis, liquid chromatography, treatment of wastewater, recovery of oil and in polymeric dyes. It has been used in applications as diverse as papermaking and biomedical research, but its use in the modification of cotton for salt-free dyeing as not been previously reported. Interest in PVAmHCl arises from the presence of a large number of cationic sites (NH+3Cl-). Nucleophilic sites involving primary amino groups within the PVAmHCl molecule are of particular value for achieving salt-free dyeing of cotton with reactive dyes. As the pH increases, the proportion of NH+3Cl- groups in the molecule decreases and that of the NH2 groups increases (Georgieva and Pishev 2001). Previous studies have shown that a variety of compounds maybe effective in this way, all involving chemical modification of cellulose. The purpose of the present study was to investigate the effects of the characteristics of the cationic agent and the pretreatment conditions on dye ability of reactive dye fibre modification technique based on polyacrylamide.
Materials and Methods
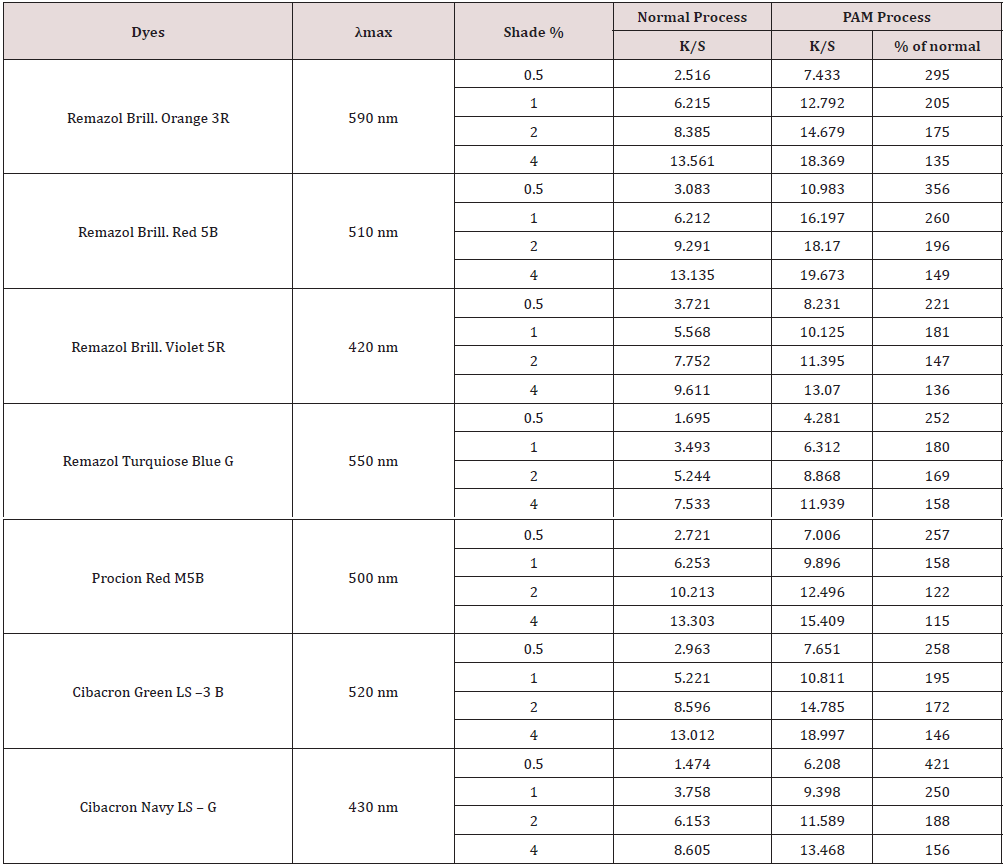
Desized, scoured and bleached 100% cotton fabric (weight 229.06 g/m2, yarn size 21.9 ×13 Ne, 99.4picks/inch and 55.4ends/ inch) and reactive dyes- Remazol Brilliant Orange 3 R, Remazol Brilliant Red 5B, Remazol Brilliant Violet 5R, Remazol Turquiose Blue G, Procion Red M5B, Cibacron Green LS–3 B, Cibacron Navy LS–G were used in present study. Acetic acid was used as a catalyst to dissolve polyacrylamide into water. Sodium sulfate (Na2SO4) was used as a salt to neutralize negative repulsive forces between the fabric surface and dye molecules and Sodium carbonate (Na2CO3) was used to facilitate the fixation of dye molecules on the fabric. Amount of PAM required for matching a shade dyed by typical process with different dyes (2% owf) (Table 1).
Preparation of Fabric
The grey fabric was designed using 2% enzyme under slightly acidic pH at 60-70°C for 2h in a laboratory jigger. The enzyme was deactivated by boiling it at 95°C for 30min and the degraded starch products were thoroughly washed out. The fabric was scoured in jigger at boiling temperature with 3% NaOH, 2% Na2CO3 and 0.5% non-ionic wetting agent for 2h and then given a hot wash followed by cold wash. The fabric was bleached with 2 volume hydrogen peroxide at 85-95°C in jigger using pH 10.5-10.8, buffered with sodium hydroxide and stabilized with sodium silicate for 2 h. Finally, the fabric was given a wash, neutralized with 0.5% sulphuric acid and washed thoroughly.
Cationization
Numerous studies have sought to improve the affinity of anionic dyes toward cotton fabric by introducing positively charged sites on cotton. This process is called cationization. The introduction of positively charged sites enables the formation of an electrostatic attraction between the fiber and the negatively charged dye molecules, thus eliminating the need for electrolytes in the cotton dyeing process and increasing the dye exhaustion and color yield of the fabric. Complete exhaustion can be achieved on cationized cotton can also be dyed with reactive dyes at neutral pH, resulting in improved dyeing compared to conventional dyeing method cationized cotton without the addition of salt.
Pretreatment with Polyacrylamide
Pad the material with calculated quantity of polyacrylamide and water with 70% expression. After padding the material is dried at ambient temperature and then cured at 120°C for 7min.
Normal Dyeing
The bleached fabric was dyed with reactive dyes (Remazol Brilliant Orange 3R) for 2% shade. The laboratory dyeing machine Rota dyer with the M:L ratio of 1:20 was used throughout the study. The fabric was kept at room temperature. One gpl non-ionic surfactant (Alphox 200), 50gpl Glaubour salt, 2gpl NaOH and 10gpl soda ash were added in the bath and the dyeing was performed for 90min at 60°C. The fabric was rinsed 3 times for 20min each, neutralized with 2.5gpl acetic acid for 30min and hot soaped at 60°C for 30min.
Dyeing of Cationized Cotton
Set the bath with calculated amount of dye solution and water. Enter the pretreated fabric into the bath. Raise the temperature to a specified level at 1.5oC / min, and dyeing continue at the set temperature for the further 60 min. Finally take out the material, soaped thoroughly and washed with cold water and dried. No salt and alkali were used while dyeing.
Determination of Exhaustion
The optical density of dye solution before and after the dyeing was measured using spectrophotometer at the maximum wavelength of absorbance (λmax). The dye bath exhaustion percentage (%E) was calculated using the following equations:
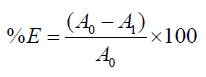
Where, Ao and Al are the absorbencies at maximum wavelength (λmax) of dye originally in the dye bath and of residual dye after dyeing respectively.
Measurement of Color Strength
Color strength K/S was measured on a Minolta Spectrophotometer. These values are calculated using the following Kubelka-Munk equation:

where, K is the absorption co-efficient, R is the reflectance of the dyed sample and S is the scattering co-efficient at the wavelength of maximum absorption.
Assessment of Fastness Properties
ISO 105-C06:2010 method on launder-O-meter was used to assess the wash fastness. The change in color and degree of staining were evaluated using geometric grey scales. The light fastness was evaluated with ISO 105-B02:2013 method on light fastness tester and the rub fastness was evaluated with ISO 105-x12:2002 on crockmaster.
Results and Discussion
Color fastness to washing, light and rubbing
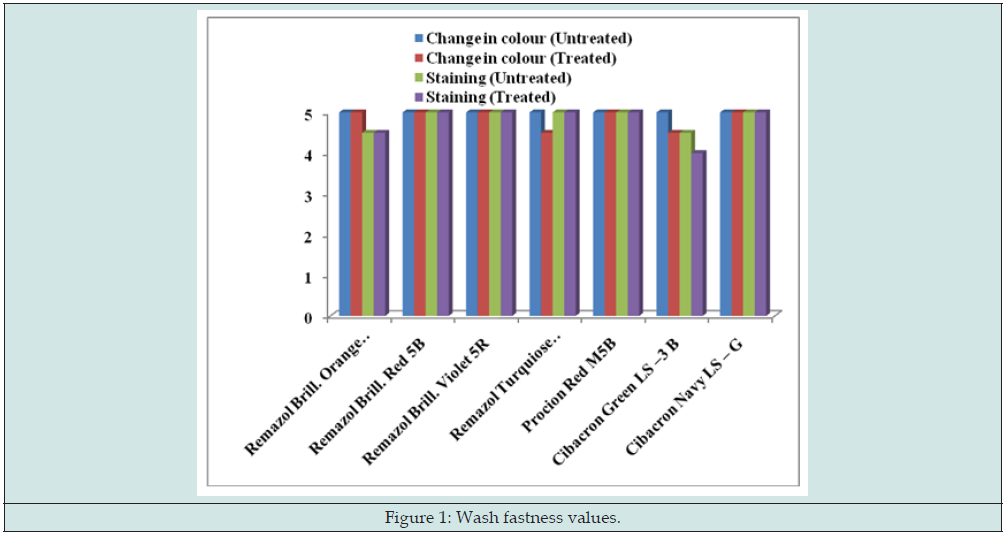
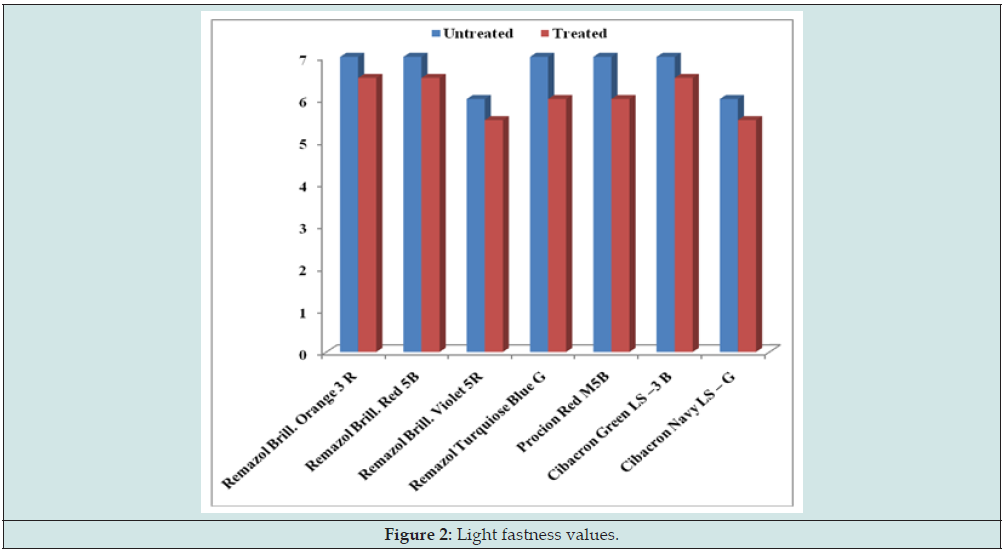
The changes in color of the dyed samples were assessed with grey scale (Figure 1), as per the recommendation of the ISO 105 method. The fastness to sunlight was determined according to ISO: 686-1985 method. The samples were exposed to daylight in an exposure rack, every day from sunrise to sunset, keeping the exposure racket an angle of 45° and the total exposure time being of 48h. The changes in color of the exposed portion were compared with those of the unexposed portion using the grey scale (Figure 2). Fastness to rubbing means the resistance of textile material to every type of rubbing and staining from the textiles in actual use. The fastness to rubbing (Figure 3) was carried out according to IS: 776-1986 method.
Color strength (K/S)
The K/S value of the untreated sample was comparatively lower than the K/S value of samples treated with different percentage of polyacrylamide. The K/S value was found to be less than that of the conventional method when 2-4% polyacrylamide was used. Moreover, the maximum K/S value found with 4% polyacrylamide and as the shade percentage of polyvinylamine chloride decreased, then K/S value decreased. Above result k/s value indicate that PAM process is better than normal process (Table 2).
Cost Consideration
The steam consumption of PAM process is only 40% of that of normal dyeing. Hence, the capacity of boiler and corresponding investment can be reduced to a sizeable volume. It is also evident that the effluent discharged in cationic dyeing process is only half to that of normal dyeing. The water purchase cost, lobour cost, chemical treatment cost and the interest on the investment on the effluent treatment plant (ETP) are greatly reduced which compensate the additional cost involved in process (Figure 4).
Cotton textiles are dyed mostly with reactive dyes because they produce a wide gamut of bright colours with excellent colour fastness to washing. However, the reactive dyeing requires considerable quantities of inorganic salt and alkali for efficient utilization and application of dyes. These salts and alkalis when drained to effluent generate heavy amounts of total dissolved solids leading to environmental pollution [9]. It has been found that pretreatment of cotton before dyeing can offer a simple and effective method of improving dye-fibre affinity, avoiding the need for salt as an electrolyte in the dye bath. It has been found that poly (vinylamine chloride) [PVAmHCl] is a physical modifying agent. Its wide range of properties has found use in catalysis, chelating, liquid chromatography, and treatment of wastewater, recovery of oil and in polymeric dyes. Previous studies have shown that a variety of compounds may be effective in this way, all involving chemical modification of Cellulosic [10,11]. Non-reactive pretreatments including some polymers with affinity for cellulose tend to be desorbed during dyeing and inhibit uptake of dye or cause it to precipitate. Recent work has established the value of polymeric quaternary ammonium compounds, amines or amides, which may be attached to cotton by non-chemical mechanisms.
Poor color fastness properties were achieved using Fe salt hard water and presence of Fe is deleterious for level dyeing of any color and shade. Poor color fastness properties were achieved using Fe salt hard water and presence of Fe is deleterious for level dyeing of any color and shade [4,9]. The percentage contribution of PAM is 7.75% which is the second dominant factor that influences the total dye utilization (T%). This may be due to its minimum utilization in the cellulose which is well supported by very low nitrogen content (0.19%). From the literature, it is known that the maximum theoretical nitrogen add-on on cellulose with PAM is 19.362% owf. But only 1% of it is utilized and the remaining seems to be hydrolysed. In the selected range, more than sufficient quantity of cationizing agent is available hence its variation within this range does not affect the response much. The optimized recipes were taken, and the bulk trials were carried out in laboratory winch dyeing machine with M:L ratio of 1:20. The colour builds up in terms of exhaustion and fixation. The exhaustion of most of the dyes are above 92% that leaves the dye bath nearly colourless, about 23% more than that of normal dyeing. Fixation is also increased by 7-9%. The total dye utilization ratio is increased to 94 % in PAM processes respectively.
Hence, ~30 % dye can be saved by using the cationization dyeing. A similar trend is also observed in some other dyes in terms of K/S value before soaping for varying depth of shade. The increase in colour strength for a particular shade varies between the dyes which may be attributed to the different dye chemistry. However, for all the dyes, there is a larger increase in light shades than in dark shades. This may probably be due to the insufficient number of cationic sites in the fibre to accommodate those larger quantities of dyes. This hypothesis is well supported by the fact that the K/S values of PAM process are greater than the corresponding K/S values of typical process. The wash fastness, light fastness and rub fastness are not affected significantly. This may be due to the formation of strong ionic bond between the fibre and the dye because it is equally good as the covalent bond that normally links the dye and fibre. The light fastness rating is slightly reduced in some dyes, about half to one point as reported by various researchers previously. The presence of an aliphatic molecule between dye and fibre may be disturbing the stable electronic configuration of dye that leads to the shifting of electrons to the higher energy state and subsequent disintegration of dye by the photons of light rays. The PAM contains primary amino groups, with which theoretically, a reactive dye should be able to react under neutral / acidic pH conditions. It is also decided to examine whether or not the PAM could, under appropriate PH conditions, assume a positive charge and so permit ‘’salt-free’’ dyeing. Pretreatment of cotton with PAM enhances the possibility of dyeing cotton at neutral pH with various commercial reactive dyes and such pretreatment also brings about some chemical changes in the treated fabric. Fastness properties are adequate and quite comparable with conventionally dyed samples. The bending resistance of the dyed fabric also improves.
The dyeing of cotton with reactive dyes using PAM with cross -linker in the dye bath improves the dye ability of cellulosic fabrics with reactive dye, when dyeing the modified substrates; reactive dyes can be much more efficiently exhausted and fixed onto cellulosic fabrics under neutral conditions in the absence of salt and alkali. The modifications show an overall suitability for different reactive dyes. The modified dyeing does not suffer either from a significant drop in colour strength, wash fastness or from duller shades. Cotton treated with PAM provides cationic dye sites and thus can be dyed with reactive dyes without electrolyte and alkali in the bath with excellent colour yield. The fastness for cationized dyeing is almost equal to that for conventional dyeing of cotton. The dyeing procedure is shorter and uses less water, chemical auxiliaries and energy than the corresponding procedure of untreated cotton. The TDS in the effluent is greatly reduced in cationic dyeing. The limitations of cationic cotton dyeing are the requirement of strict process control, liberation of ammonia vapour in the dye house and reduction of light fastness in some dyes. So, PAM can be a potential solution for salt and alkali free reactive dyeing if this study is further increased to achieve best fastness values by varying the pretreatment criteria or dyeing procedure of cationised cotton. Cationization with cationic agent requires more extra pretreatment step which may lead extra cost. So use of cationic reactive dye may have possibilities to catch future market due to growing concern for pollution free dyeing. By using this pretreatment method, the following advantages were observed: Elimination of salt as an electrolyte, Maximum fixation of dye, Minimum hydrolysis of dye, Low volume of water requirement during the wash-off process, Significant savings in process costs and environmentally friendly. Thus the purpose of present study was to give an overall idea about the pre-treatment of cotton with a PAM could enhance the dye ability of the fibre with reactive dyes which helps the further research in this area.
References
- Broadbent AD (2005) Water treatment. Basic Principles of Textile Coloration. AD Broadbent (Ed.,), First ed: Society of Dyers and Colourists, pp. 130-151.
- Bide M (2007) Environmentally responsible dye application. Environmental Aspects of Textile Dyeing. RM Christie (Eds.,) First ed, Woodhead Publishing Ltd, pp. 74-92.
- Khatri A (2011) Use of Biodegradable Organic Salts for Pad-Steam Dyeing of Cotton Textiles with Reactive Dyes to Improve Process Sustainability. International Conference on Education, Research and Innovation, IPEDR vol.18 (2011) © (2011) IACSIT Press, Singapore.
- Matsui U, Zollinger H (1988) Dye Fiber Bond Stabilities of Dyeings of Bifunctional Reactive Dyes Containing a Monochlorotriazine and a Beta-Hydroxyethylsulfone Sulfuric-Acid Ester Group. J Soc Dye & Col 104(11): 425-431.
- Aktek T, Millat AKMM (2017) Salt Free Dyeing of Cotton Fiber- A Critical Review. Inter J Textile Sci 6(2): 21-33.
- Chattopadhyay D (2001) Cationization of cotton for low-salt or salt-free dyeing. Indian J Fibre and Textile Res 26(1/2): 108-115.
- Khatri A (2015) A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J Cleaner Production 87: 50-57.
- Choudhury RA (2014) Coloration of Cationized Cellulosic Fibers-A Review. AATCC J Res 1(3): 11-19.
- Saleem F, Amin S (2017) Study of Cotton Fabric Dyeing by Reactive Dyes in Various Water Hardness Systems. J Chem Soc Pak 39(1): 6-10.
- Periyasamy AP, Dhurai B, Thangamani K (2011) Salt-free dyeing- A new method of dyeing on lyocell/cotton blended fabrics with reactive dyes. Autex Res J 11(1): 14-17.
- Georgieva A, Pishev D (2001) Dyeing of Cellulose Textile Materials with Mono - And Polyfunctional Reactive Dyes. Journal of the University of Chemical Technology and Metallurgy, XXXVI, Book 2, - Sofia, Bulgaria.87

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...