Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
The Prevalence of Hypothyroidism in Patients with Type 2 Diabetes Mellitus in Saudi Community based Hospital a Retrospective Single Centre Study Volume 2 - Issue 1
Khalid S Aljabri1*, Samia A Bokhari1, Muneera A Alshareef1, Patan M Khan1, Abdulla M Mallosho1, Hesham M AbuElsaoud1, Mohammad M Jalal1, Rania F Safwat1, Rehab El Boraie1, Nawaf K Aljabri2 and Bandari K Aljabri3
- 1 Department of Endocrinology, Jeddah, Kingdom of Saudi Arabia
- 2 Department of Laboratory, Haffr albatin, Kingdom of Saudi Arabia
- 3 College of medicine, Makkah, Kingdom of Saudi Arabia
Received:January 22, 2019; Published: January 28, 2019
Corresponding author:Khalid S. Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
DOI: 10.32474/ADO.2019.02.000126
Abstract
Background and Objective
The association between diabetes and thyroid dysfunction were studied. To estimate retrospectively the frequency of hypothyroidism in patients with type 2 diabetes (T2DM) in Saudi community-based hospital.
Design
We analyzed retrospectively 3760 participants whom are between the age 20 to 98 years. All patients were from the population of the Primary health centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. All data were collected on the basis of a review of electronic medical data. Patients with TSH above the normal range of TSH for our laboratory reference (4.2 MIU/L, history of hypothyroidism and taking thyroid replacement therapy were included. Patient who are pregnant were excluded.
Results
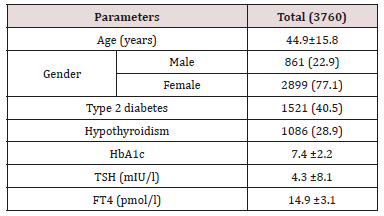
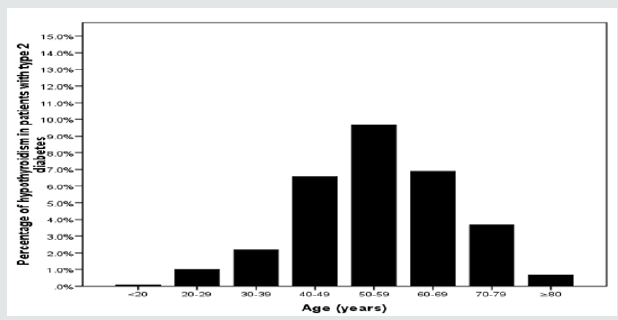
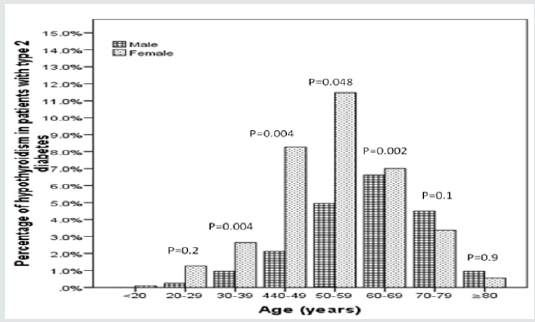
Out of the initial screening of 6023 subjects, 3760 subjects were included. There were 861 (22.9%) male and 2899 (77.1%) were female with mean age 44.9±15.8. The mean TSH value was 4.3±8.1. Among them we found 1521 (40.7%) and 1086 (28.9%) cases with T2DM and hypothyroidism respectively. Among cases of T2DM, there were 467 (30.7%) with hypothyroidism. There were 86 (18.4%) cases were male and 381 (81.6%) were female with male to female ratio of 1 to 4.4, p< 0.0001. Cases with hypothyroidism were nonsignificantly older than cases with no hypothyroidism, 55.2±12.6 vs. 54.9±13.2 respectively, p=0.6. Cases with hypothyroidism were nonsignificantly showed no differences in HbA1c than cases with no hypothyroidism, 7.9±2.3 vs. 8.1±2.1 respectively, p=0.2. Hypothyroidism was more prevalent in the sixth decade (32%), figure 1. Hypothyroidism was significantly more prevalent in females between the third to the seventh decades as compared to males. Male was no significantly more prevalent than females in the eighth and ninth decades.
Conclusion
We conclude that despite the limitations of this hospital-based retrospective study, hypothyroidism is highly prevalent in cohort of Saudis with T2DM. The majority of our patients with primary hypothyroidism were predominantly females. These two observations remain to be validated by population-based studies. In the absence of registry data, larger cooperative studies involving diverse population samples from multiple centers could help to provide further information on the true frequency nationally. Based on a high prevalence of hypothyroidism among Saudi T2DM patients, routine screening for hypothyroidism is highly recommended in Saudi diabetic population.
Keywords: Hypothyroidism; Type 2 Diabetes; Saudi Arabia
Introduction
Thyroid gland is one of the important organs in human body and the burden of thyroid diseases in the general population is enormous specially in females [1,2]. Thyroid dysfunctions have increased recently and are considered the commonest endocrine diseases [3]. Several studies have been reported from different parts of the world showing the prevalence of hypothyroidism. The prevalence of spontaneous hypothyroidism is between 1% and 2%, and it is more common in older women and ten times more common in women than in men [4]. Diabetes Mellitus is the commonest endocrine disorder, leading cause of death worldwide [5]. The World health organization estimated diabetes prevalence was 2.8% in 2000 and 4.4% in 2030. The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030 [6]. Saudi Arabia is the seventh of the top ten countries in terms of the prevalence of diabetes among the adult population aged 20-79 [7].
The association between diabetes and thyroid dysfunction were first published in 1979 [8]. The strong link between diabetes and thyroid diseases encouraged the American Diabetes Association (ADA) to propose that people with diabetes must be checked periodically for thyroid dysfunction [9]. Thyroid disease should be screened annually in diabetic patients to detect asymptomatic thyroid dysfunction [10]. A number of studies estimated prevalence of thyroid dysfunction among diabetes patients ranging from 2.2-17% [11-13]. However, fewer studies have estimated higher prevalence of thyrodiabetics i.e. 31% and 46.5% respectively. [14,15] The prevalence of thyroid dysfunction among Saudi diabetic patients was reported to be 16-28.5% of which 25.3% had hypothyroidism [16,17]. Epidemiological studies of thyroid dysfunction have limitations, for example the definition of overt hypothyroidism and subclinical hypothyroidism, the selection criteria of the sample used, the influence of age, sex, genetic and environmental factor and the different techniques used for the measurement of thyroid hormones and the relative paucity of incidence data. [5] Thus, the present study was conducted to find out the relationship between type 2 DM and hypothyroidism in patients with type 2 diabetes (T2DM) in a cohort of Saudi population.
Methods
We analyzed retrospectively 3760 participants whom are between the age 20 to 98 years. All patients were from the population of the Primary health centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. All data were collected on the basis of a review of electronic medical data. Patients with Thyrotropin level (TSH) above the normal range of TSH for our laboratory reference, history of hypothyroidism and taking thyroid replacement therapy were included. Patient who are pregnant were excluded. The study designed to investigate hypothyroid in T2DM by estimation of TSH among Saudi adult males and females to determine the prevalence hypothyroidism in studies population in order to compare the result to other population worldwide. The reference range values of TSH 0.22-4.2 MIU/L, Free T4 12.0-22.0 pmol/L. Participants were defined as having T2DM according to self-report, clinical reports, use of antidiabetic agents and HbA1c (≥6.5). [18] HbA1c was expressed as percentage. High performance liquid chromatography was used. The total number of cohorts were separated on basis of age values into eight groups: <20 years, 20-29 years, 30-39 years, 40-49 years, 50-59 years, 60-69 years, 70-79 years and ≥80 years.
Statistical Analysis
Continuous variables were described using means and Standard Deviations. Univariate analysis of baseline demography both between groups, were accomplished using unpaired t-test and Chi square test were used for categorical data comparison. P value <0.05 indicates significance. The statistical analysis was conducted with SPSS version 22.0 for Windows.
Results
Table 2: Base line characteristic and comparison between patients with type 2 diabetes with and without hypothyroidism.
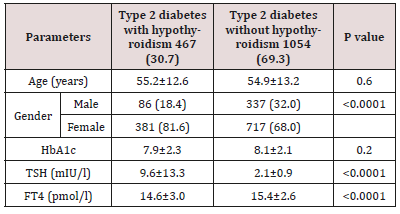
Out of the initial screening of 6023 subjects, 3760 subjects were included. There were 861 (22.9%) male and 2899 (77.1%) were female with mean age 44.9±15.8 (Table 1). The mean TSH value was 4.3±8.1. Among them we found 1521 (40.7%) and 1086 (28.9%) cases with T2DM and hypothyroidism respectively. Among cases of T2DM, there were 467 (30.7%) with hypothyroidism (Table 2). There were 86 (18.4%) cases were male and 381 (81.6%) were female with male to female ratio of 1 to 4.4, p< 0.0001. Cases with hypothyroidism were nonsignificantly older than cases with no hypothyroidism, 55.2±12.6 vs. 54.9±13.2 respectively, p=0.6. Cases with hypothyroidism were nonsignificantly showed no differences in HbA1c than cases with no hypothyroidism, 7.9±2.3 vs. 8.1±2.1 respectively, p=0.2. Hypothyroidism was more prevalent in the sixth decade (32%) (Figure 1). Hypothyroidism was significantly more prevalent in females between the third to the seventh decades as compared to males (Figure 2). Male was nonsignificantly more prevalent than females in the eighth and ninth decades (Figure 2).
Discussion
The current study revealed that hypothyroidism was found in 30.7% in patients with T2DM. The association between diabetes and thyroid disease is well known. Thyroid diseases are also common in the general population. Diseases of the thyroid are of great importance because most are amenable to medical management. There is a great difference in the prevalence of the thyroid diseases in the general population, ranging from6.6% to 13.4% [19,20]. Large scale studies of thyroid dysfunction in the United States have reported prevalence of 6.6% in the general population [21,22]. The first studies were published in year 1997 [23]. Therefore, several studies in different countries were conducted to estimate the prevalence of thyroid disease in diabetic patients. Some studies estimated the prevalence to be 10–24% [24]. Our finding is more than that has been reported by Akbar et al. [16] and Al-Geffari et al. [17] of 16% in 2006 and 25.3% in 2013 respectively. In this study, we report the highest prevalence of thyroid dysfunction in T2DM patients when compared with other communities, shown by the Scotland study to be 13.4% among both Type 1 diabetes and T2DM or by the Jordanian study, where it was 12.5% among Type 2 diabetes. [11,13] Studies from Libya and Oman showed the prevalence of hypothyroidism to be 2.3% and 406% respectively [25]. A meta-analysis data in a study of 10920 patients with diabetes which revealed a mean frequency of thyroid disease of 11% [26].
In general population, it was documented by many authors that hypothyroidism is more common in females [27-32]. In the present study, the prevalence of thyroid disorders was more in females as compared to males (81.6% versus 18.4%). This was statistically significant, P value of < 0.0001. Our results are consistent with studies of Celani, et al. [14], Papazafiropoulou et al. [23], Babu et al. [33] and Michalek et al. [34], Vondra, et al. [35] but our findings seemed to be relatively higher [36]. Thus, the prevalence of thyroid disorders in diabetic patients is influenced by female gender. Interestingly, the present study observed nonsignificant higher in TSH among T2DM females in compression with males, 10.0±14.3 vs. 7.7±6.8, p=0.1. In concurrent with previous finding that, estrogen hormone has direct role in thyroid follicular cells, and its role in thyroxine binding globulin (TBG) [16], explain higher level of TSH and FT3 in T2DM females [25,37].
It is reported that DM appears to influence thyroid function in at least two sites, one at the level of hypothalamic control of TSH release and the other at the conversion of thyroxine to triiodothyronine in the peripheral tissues [38]. Perros et al. [11] reported that “the thyroid hormones, triiodothyronine and tetraiodothyronine are insulin antagonists that also potentiate the action of insulin indirectly.” TRH synthesis decreases in diabetes, and this could be responsible for the occurrence of low thyroid hormone levels in diabetics. In the literature, it is well known that thyroid hormones directly control insulin secretion, thus affecting the control of diabetes. In hypothyroidism, there is a reduction in glucose induced insulin secretion by beta cells, and the response of beta cells to glucose or catecholamine is increased in hyperthyroidism due to increased beta cell mass. Moreover, insulin clearance is increased in thyrotoxicosis. [39,40] However, in our study, we failed to establish the association between T2DM with hypothyroidism and T2DM without hypothyroidism represented by HbA1c, 7.9±2.3 and 8.1±2.1 respectively, p value 0.2. This can be explained by the other various factors that can play role in the control of diabetes including patients lifestyle, compliance to treatment, other used medications, and the duration of diabetes and thyroid disease.
Primary hypothyroidism occurs in all ages, but it is usually more prevalent, in both community- and hospital-based populations, in older people in their sixth and seventh decades. [28,32] Out of 1521 diabetic patients who had hypothyroidism, 31.5% of patients had age 50-59 years. Thus, the prevalence of thyroid disorder was found to be lower in patients with more age. The results of present study are in disconcordance with the previous studies of Feely, et al. [8], Michalek, et al. [34], Whitehead, et al. [40], Vondra, et al. [41], Moulik, et al. [42] and Johnson et al, who also found high prevalence of thyroid disorders in diabetic patients with advancing age [43].
We aimed to identify the frequency of hypothyroidism in patients with T2DM in primary health care setting. Furthermore, due to the retrospective nature of this study, the observed population reflects a selected yet comprehensive group of patients rather than the general population. Our study could be limited by the question of clustering of cases within the study region and the effect that might have on our estimates, in addition, the current study population may appear limited in size and therefore may underestimate the true frequency of hypothyroidism in patients with T2DM. In addition, the study shares the limitations of all retrospective studies.
We conclude that despite the limitations of this hospitalbased retrospective study, hypothyroidism is highly prevalent in cohort of Saudis with T2DM. The majority of our patients with primary hypothyroidism were predominantly females. These two observations remain to be validated by population-based studies. In the absence of registry data, larger cooperative studies involving diverse population samples from multiple centers could help to provide further information on the true frequency nationally. Based on a high prevalence of hypothyroidism among Saudi T2DM patients, routine screening for hypothyroidism is highly recommended in Saudi diabetic population.
References
- LaFranchi S (1994) Adolescent thyroid disorders. Adolesc Med 5(1): 65- 86. In: Memonab A. Family history of benign thyroid disease and cancer and risk of thyroid cancer. Eur J Cancer 40: 754-760.
- Okosieme OE, Marx H, Lazarus JH (2008) Medical management of thyroid dysfunction in pregnancy and the postpartum. Expert Opin Pharmac other 9(13): 2281-2293.
- Madariaga AG, Palacios SS, Guillén Grima F, Galofré J (2014) The incidence and prevalence of thyroid dysfunction in europe: a metaanalysis. J Clin Endocrinol Metab 99(3): 923-931.
- Vanderpump MP (2005) The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. JB Lippincott-Raven, Philadelphia 9: 398-406.
- Faghilimnai S, Hashemipour M, Kelishadi B (2006) Lipid profile of children with type 1 diabetes compared to controls. ARYA J 2(1): 36-38.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5): 1047-1053.
- International Diabetes Federation (2012) IDF Diabetes Atlas 5th edition update.
- Feely J, Isles TE (1979) Screening for thyroid dysfunction in diabetics. Br Med J 1(6179): 1678.
- American Diabetes Association (2019) “Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019.” Diabetes Care 42 (Supplement 1): S34-S45.
- RS Gray, WJ Irvine, BF Clarke (1979) “Screening for thyroid dysfunction in diabetics,” British Medical Journal 2(6202): 1439.
- Perros P, McCrimmon RJ, Shaw G, Frier BM (1995) Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med 12(7): 622-627.
- Smithson MJ (1998) Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med 15(2): 148-150.
- Radaideh AR, Ajlouni KM, Amari FL, Bateiha AE, El Khateeb MS, et al. (2004) Thyroid dysfunction in patients with type 2 diabetes mellitus in Jordan. Saudi Medical Journal 25(8): 1046-1050.
- Celani MF, Bonati ME, Stucci N (1994) Prevalence of abnormal thyrotropin concentrations measured by a sensitive as say in patients with Type 2 diabetes mellitus. Diabete Res 27(1): 15-25.
- Udoing CEJA, Udoh E, Etukudoh ME (2007) Evaluation of thyroid function in diabetes mellitus in Calabar, Nigeria. Indian J Clin Biochem 22(2): 74-78.
- DH Akbar, MM Ahmed, J Al Mughales (2006) “Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics.” Acta Diabetologica 43(1): 14-18.
- Metab Al Geffari, Najlaa A Ahmad, Ahmad H Al Sharqawi, Amira M Youssef, Dhekra AlNaqeb, et al. (2013) “Risk Factors for Thyroid Dysfunction among Type 2 Diabetic Patients in a Highly Diabetes Mellitus Prevalent Society.” International Journal of Endocrinology 2013:1-6.
- American Diabetes Association (2019) Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 42 (Supplement 1): S13-S28.
- RC Silva (2005) “Importancia da avaliacao da funcao tireoidiana em pacientes com diabetes mellitus.” Arquivos Brasileiros de Endocrinologia & Metabologia 49(2): 180-182.
- GE Umpierrez, KA Latif, MB Murphy, Helen C Lambeth, Frankie Stentz, et al. (2003) “Thyroid dysfunction in patients with type 1 diabetes.” Diabetes Care 26(4): 1181-1185.
- GJ Canaris, NR Manowitz, G Mayor, EC Ridgway (2000) “The colorado thyroid disease prevalence study.” Archives of Internal Medicine 160(4): 526-534.
- JG Hollowell (2002) “Serum TSH, T4, and thyroid antibodies in theUnited States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III).” Journal of Clinical Endocrinology and Metabolism 87(2): 489–499.
- A Papazafiropoulou, A Sotiropoulos, A Kokoloki, M Kardara, P Stamataki (2010) Prevalence of thyroid dysfunction among greek type 2 diabetic patients attending an outpatient clinic.” Journal of Clinical Medicine Research 2(2): 75-78.
- H Gharib, RM Tuttle, J Baskim, LH Fish, PA Singer, et al. (2004) “Consensus statement. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association and the Endocrine Society,” Endocrine Practice 10(6): 497-501.
- Ashraf Ahmed, Siddig B Mohamed, Salih A Elmadi, Abdelkarim A Abdorabo, Ismail M Ismail, et al. (2017) Assessment of Thyroid Dysfunctions in Type 2 Diabetes Mellitus Patients in Surman, Western- Libya. International Journal of Clinical and Experimental Medical Sciences 3(1): 1-4.
- R Kadiyala, R Peter, OE Okosieme (2010) “Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies.” International Journal of Clinical Practice 64(8): 1130-1139.
- Tunbridge WMG, Evered DC, Hall R, Appleton D, Brewis M, et al. (1977) The spectrum of thyroid disease in the community: the Whickham survey. Clin Endocrinol 7(6): 481-493.
- Dos Remedios LV, Weber PM, Feldman R, Schurr DA, Tsoi TG (1980) Detecting unsuspected thyroid dysfunction by the free thyroxine index. Arch Intern Med 140(8): 1045-1049.
- Riniker M, Tieche M, Lupi GA, Grob P, Studer H, et al. (1981) Prevalence of various degrees of hypothyroidism among patients of general medical department. Clin Endocrinol 14(1): 69-74.
- Schectman JM, Kallengerg GA, Shumacher RJ, Hirsch RP (1989) Yield of hypothyroidism in symptomatic primary care patients. Arch Intern Med 149(4): 861-864.
- Bastenie PA, Bonnyns M, Vanhaelst L (1985) Natural history of primary myxedema. Am J Med 79(1): 91-100.
- Tunbridge WMG, Brewis M, French JM, et al (1981) Natural history of autoimmune thyroiditis. Br Med J 282(6260): 258-262.
- Babu K, Kakar A, Byotra SP (2001) Prevalence of thyroid disorder in type II diabetes mellitus patients. J Assoc Phys Ind 49: 43.
- Michalek AM, Mahoney MC, Calebaugh D (2000) Hypothyroidism and diabetes mellitus in an American Indian population. J Family Practice 49(7): 53-55.
- Vondra K, Vrbikova J, Dvorakova K (2005) Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol 30(4): 217- 236.
- Ana Paula Santin, Tania weber Furlametto (2011) Role of Estrogen in Thyroid Function and Growth regulation. Journal of Thyroid Research, p. 1-7.
- Y Suzuki, M Nanno, R Gemma, I Tanaka, T Taminato, et al. (1994) “The mechanism of thyroid hormone abnormalities in patients with diabetes mellitus.” Nippon Naibunpi Gakkai zasshi 70(4): 465-470.
- P Mitrou, SA Raptis, G Dimitriadis (2010) “Insulin action in hyperthyroidism: a focus on muscle and adipose tissue.” Endocrine Reviews 31(5): 663-679.
- S Stanicka, K Vondra, T Pelikanova, P Vlcek, M Hill, V Zamrazil (2005) “Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy.” Clinical Chemistry and Laboratory Medicine 43(7): 715–720.
- Whitehead C, Lunt H, Pearson JF, Cawood TJ (2010) Is screening for hypothyroidism in the diabetes clinic effective? Practical Diabetes 27(3): 113-117.
- Vondra K, Vrbikova J, Dvorakova K (2005) Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol 30(4): 217- 236.
- Moulik PK, Nethaji C, K haleeli AA (2002) Thyroid dysfunction in diabetes: can we justify routine screening? Endocrine Abstracts 3: 292.
- Johnson JL (2002) Diabetes and thyroid disease: a likely combination. Diabetes Spectrum 15(3): 140-142.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...