
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
The Effect of Photobiomodulation Therapy on Energy Supply and Functional Activity of Leukocytes from Rats with Experimental Diabetes Mellitus Volume 3 - Issue 5
Oleksandr Karmash*, Mariana Liuta, Nataliia Yefimenko and Nataliia Sybirna
- Department of Biochemistry, Faculty of Biology, Ivan Franko National University of Lviv, Ukraine
Received:December 25, 2021; Published:January 12, 2022
Corresponding author:Oleksandr Karmash, Department of Biochemistry, Faculty of Biology, Ivan Franko National University of Lviv, 4, Hrushevskyi St, Lviv 79005, Ukraine
DOI: 10.32474/ADO.2022.03.000174
Abstract
Background: Impairing in leukocytes function is the main reason of greater susceptibility to infections observed in patients with diabetes mellitus. The distinctive feature of diabetes is dysregulation in glucose distribution in different tissues and cells in organism. There are evidence that downregulation of glucose transport and lack of energy in leukocytes may contribute to the detriment of their functions. We investigated possible positive effects of photobiomodulation therapy on energy supply and functional activity of leukocytes from rats with experimental diabetes mellitus.
Methods: The experiments were conducted using male Wistar rats. Fractioning of blood leukocytes was performed in gradient of ficollsodium amidotrizoate density. Energy supply of leukocytes was evaluated using 2-NBDG (2-deoxy-2-((7-nitro-2,1,3-benzoxadiazol- 4-yl) amino)-D-Glucose) staining for glucose uptake screening and ATP assay kit for ATP concentration measurement. Functional activity of leukocytes was investigated using measurement of ROS concentration (with H2DCFDA staining), myeloperoxidase, phagocytic and bactericidal activity.
Results: Progression of experimental diabetes mellitus was accompanied by decrease in leukocyte glucose uptake, ATP concentration, myeloperoxidase activity, phagocytic and bactericidal activity. Treatment with photobiomodulation therapy promotes the restoration of these downregulated indices, resulting in normalization of leukocytes functions.
Conclusions: Photobiomodulation therapy has significant positive effect in restoration of energy supply and functional activity of leukocytes from rats with experimental diabetes mellitus
Keywords:Photobiomodulation Therapy; Diabetes Mellitus; Leukocytes; Glucose Uptake
Abbreviations:2-NBDG: 2-deoxy-2-((7-nitro-2,1,3-benzoxadiazol-4-yl) amino)-D-Glucose; AGEs: Advanced Glycosylation End products; EDM: Experimental Diabetes Mellitus; H2DCFDA: 2’,7’-Dichlorodihydrofluorescein Diacetate; HIF-1α: Hypoxia-Inducible Factor 1α; IFP: Index of Finished Phagocytosis; MCC: Mean Cytochemical Coefficient; NBT: Nitroblue Tetrazolium; OGTT: Oral Glucose Tolerance Test; OMP: Oxidatively Modified Proteins; PBM: Photobiomodulation; PI: Phagocytic Index; PMNL: Polymorphonuclear Leukocytes; RAGE: Receptor for Advanced Glycation End Products; ROS: Reactive Oxygen Species; MPO: Myeloperoxidase; SOD: Superoxide Dismutase; TBA: Thiobarbituric Acid; TNF: Tumor Necrosis Factor
Introduction
According to WHO in 2014 8.5% of adult population on Earth were recognized as diabetic. In 2016 diabetes was the direct reason of 1.6 million deaths. Diabetes mellitus (DM) is a chronic illness, which emerges due to the lack of insulin or disorders in its functioning, resulting in uncontrolled hyperglycemia. Prolonged high blood glucose concentration leads to development of oxidative stress and further complications (cardiomyopathy, retinopathy, nephropathy, etc.). One of the main features of DM is deregulation of glucose distribution in different tissues of organism. DM characterized by intensification of glucose transport in enterocytes, which is accompanied by high expression of active and facilitative transporters. Meanwhile the absorption of glucose is increased in intestine, peripheral tissues demonstrate decreased intake of glucose due to defects in translocation of transporters to plasma membrane [1]. Intensified intake of glucose in bloodstream and lower ability of peripheral tissues to absorb it from blood to cells leads to hyperglycemia. There are many research, that focus on glucose transport, because disorder in glucose metabolism leads to loss of normal cell functions. However, these works focus mainly on classic insulin-dependent tissues, such as skeletal muscles, adipocytes and β-cells of pancreas [2-5], leaving features of glucose transport in leukocytes of DM patients poorly studied. It is important to note, that information about glucose transporters in leukocytes is deficient and controversial. Enhanced susceptibility to infections and disorders in immune system are well known complications of DM. Infections in those patients are more severe and other complications are more frequent. There are publications about altered functions of leukocytes during DM, such as chemotaxis, adhesion and activity of phagocytosis. Nonetheless, scientific evidence in this field is scarce and predominantly regard polymorpho-nuclear leukocytes and lymphocytes [6,7]. It is important to note, that changes in glucose transport considered as one of the main factors defining normal functioning of leukocytes. However, those functions of immune cells during DM that are not directly connected with immune response are rarely investigated [8,9]. Recently, Photobiomodulation (PBM) therapy provokes a great interest as convenient and noninvasive type of therapy, possessing antioxidant and antihyperglycemic activity. In previous research [10] we revealed the decrease of blood glucose concentration in rats with DM under treatment with PBM. We hypothesized that this effect can be explained by partial regeneration of pancreatic β-cells and, according to this, improved ability of peripheral tissues to utilize glucose. In addition, we observed the decrease of oxidative stress markers in blood plasma and leukocytes. According to mentioned above, the objective of our study was to investigate the effect of PBM on energy supply and how it affects functional activity of leukocytes during DM.
Materials and Methods
Induction of Diabetes
Research was performed using Wistar male rats with weight 120-180 g. Animals were contained in standard vivarium conditions with free access to food and water. Manipulations with animals were conducted according to General Ethical Principles of Animal Experiments approved by the First National Congress for Bioethics (Kyiv, 2001), which agrees with the provisions of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986). Experimental diabetes mellitus (EDM) was induced by intraperitoneal injection of streptozotocin (Sigma, USA), diluted in 10 mM Na-citrate buffer (pH 4.5) calculated as 6 mg of streptozotocin per 100 g of animal weight. Two weeks after induction of EDM animals with blood glucose concentration higher than 12 mmol/L were included in experiments.
Measuring of Glucose Concentrat
Glucose concentration in whole blood was measured by glucose oxidase method using “Filicitdiagnostica” kit (Ukraine) according to manufacturer instructions. Blood samples for measurements were collected from tail vein.
Design of Experiment
Animals were divided into four groups: 1 – control animals; 2 – control animals treated with PBM therapy; 3 – animals with EDM; 4 – animals with EDM treated with PBM therapy. Rats were irradiated daily for 5 min during 10 days at anterior side of full body (area of irradiation was 42,75 cm2, delivered energy 44.5 J). A matrix of 30 ultra-bright light-emitting diodes was used as a light source with the following parameters: wavelength of 630–660 nm, total power optical output of 150 mW, irradiance of 3.47 × 10-3 W/cm2 and energy density of 1.041 J/cm2 which was measured by thermoelectric radiometer RTN-20 with the span of spectral sensitivity from 0.4 to 6 μm. The light source is an analogue of the Barva Fleks/FM photon matrices (Laser and Health Corporation, Ukraine).
Glucose Tolerance Test
Oral Glucose Tolerance Test (OGTT) was performed in morning after 12-h fasting of animals. Rats were orally injected with glucose solution in water calculated as 1 g per 1 kg of animal weight. Glucose concentration in blood was measured before (0 min) and 30, 60, 90, 120 min after injection. The level of glucose tolerance was determined by measuring area under glycemic curve using trapezoid rule [11].
Blood Collection
After 10 days of PBM therapy treatment blood was collected by decapitation of animals under ether anesthesia. To prevent coagulation, blood was collected in vials with addition of heparin (final dilution heparin : whole blood = 1:100). Portion of blood (2 ml) was centrifuged for 15 min at 3000 rpm for obtaining blood plasma (stored at -20 ℃ for further experiments). The rest of blood was used for leukocytes isolation.
Leukocyte Isolation
Leukocytes were isolated by centrifuging blood at the ficollsodium amidotrizoate density gradient (ρ = 1.076–1.078). The isolated cells were washed twice in a phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 × 7H2O, 1.8 mM KH2PO4, pH 7.4) and used in the experiments or immediately frozen with liquid nitrogen and stored at –20 °C. Leukocytes lysate was used for the experiments. Cells (2 × 106) were lysed using a 25 mM Tris-HCl buffer (pH 7.5), which contained 0.5% Triton X-100, 100 mM KCl, 5 mM MgCl2, 2 mM EDTA, and inhibitors of proteases ((“Carl Roth GmbH+Co. KG”, Germany, Art. No. 3751.1). The lysates were centrifuged for 15 min at 8000 rpm, and the obtained supernatant was used in the experiment.
Activity of Glucose Uptake by Leukocytes
Glucose uptake by leukocytes was analyzed using fluorescent microscopy with 2-NBDG (2-deoxy-2-((7-nitro-2,1,3- benzoxadiazol-4-yl) amino)-D-Glucose) staining. Freshly isolated leukocytes in the amount of 5 × 105 were incubated for 30 min at 37 ℃ in 50 μl of 0.1 M Krebs-Ringer phosphate buffer (pH 7.2-7.4) with addition of 1.25 μl of 20 mM 2-NBDG. After incubation cells were washed thrice by addition of 0.5 ml Krebs-Ringer phosphate buffer and centrifuging for 5 min at 3000 rpm. Washed leukocytes in the amount of 1 × 105 were placed on microscope glass slide, rested for 2-3 min for sedimentation and analyzed by inverted microscope Olympus IX73 equipped with DP74 digital camera. Obtained photos were analyzed using ImageJ program (v. 1.53e, NIH, USA). Activity of 2-NBDG fluorescence was expressed in arbitrary units (AU).
Measurement of ATP Content
ATP content in leukocytes lysates was measured using ATP Colorimetric/Fluorometric Assay Kit (CN MAK190, Sigma-Aldrich, USA) according to manufacturer’s instruction. 2 × 106 cells were lysed as described above. 50 μl of obtained supernatant were used for analysis. ATP content in leukocytes was expressed in ng of ATP in 1 μl of cell lysate.
Measurement of Reactive Oxygen Species (ROS) Content
ROS content in leukocytes was measured by fluorescence intensity of 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA). Freshly isolated leukocytes in the amount of 2 × 106 were incubated for 30 min in darkness at 37 ℃ in 1 ml of PBS containing 5 μM H2DCFDA. After incubation cells were washed once by addition of 0.5 ml PBS and centrifuging for 5 min at 3000 rpm. Cells in amount of 2 × 105 were placed on microscope glass slide, rested for 2-3 min for sedimentation and analyzed by Nikon Optiphot-2 microscope equipped with HB-10101AF lamp and DCM310 digital camera. Obtained images were analyzed using ImageJ program (v. 1.53e, NIH, USA). Activity of H2DCFDA fluorescence was expressed in Arbitrary Units (AU).
Measurement of Myeloperoxidase (MPO) Activity
Myeloperoxidase activity in leukocytes lysates and blood plasma was measured spectrophotometrically. To 10 μl of sample (leukocytes lysate or blood plasma) we added 290 μl of 50 mM phosphate buffer (pH 6.0) containing 0.3 mM H2O2 and 0.68 mM o-dianisidine. Enzyme activity was analyzed by changes in optical density of experimental sample at λ=450 nm for 5 min at 37 ℃. Obtained results were expressed as nmol H2O2 / min × mg of protein.
Analysis of Neutrophil Granulocytes Phagocytic Activity
Polymorphonuclear leukocytes and monocytes of peripheral blood are able to bind on their surface, intake and digest microbial test-culture. Phagocytosis object was prepared according to [12]. Before the experiment we prepared yeast suspension with concentration of 40 × 106 cells per 1 ml by adding 4 mg of fresh yeasts (Saccharomyces cerevisiae) to 4 ml of water. For phagocytosis analysis of neutrophil granulocytes there must be 5 times bigger concentration of phagocytosis objects. Yeast suspension was incubated for 5 min at 37 ℃ with stirring. To 0.4 ml of this suspension, we added 0.4 ml of 2% trypan blue solution and boiled for inactivation for 5 min at 90 ℃. After that, phagocytosis objects were washed from dye excess by adding distilled water and centrifuging for 5 min at 1500 rpm until decolorization of supernatant. Obtained sediment of dyed yeasts was resuspended in 0.4 ml of PBS and used for phagocytosis analysis of neutrophil granulocytes.
In two vials we added 50 μl of leukoconcentrate (3 × 106 cells) and 5 μl of yeast suspension. One vial was incubated at 37 ℃ for 30 min and another for 120 min. After the incubation, vials were centrifuged for 3 min at 2000 rpm. From cells sediment we prepared cell smears on microscope glass slides. Smears were dried and fixated with methanol. After complete methanol evaporation smears were covered with 70% ethanol. Gradually by droplets was added distilled water to wash ethanol. Cell smears were stained by Romanowsky-Giemsa for 40 min, washed with distilled water and dried. Smears were analyzed by counting phagocytic neutrophil granulocytes and phagocytosed particles. Phagocytic Index (PI) (percent of phagocytosis) was determined as ratio of mean number of phagocytic cells to general number of counted cells (200):
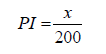
where x – number of phagocytic cells.
Index of finished phagocytosis (IFP) was determined by formula:
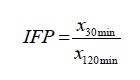
where x30min – mean number of yeast cells phagocytosed after 30 min of incubation; x120min – mean number of yeast cells phagocytosed after 120 min of incubation.
Analysis of Neutrophil Granulocytes Bactericydal Activity by Reduction of Nitroblue Tetrazolium [13]
Reaction of nitroblue tetrazolium reduction (NBT-test) gives possibility to analyze phagocytic and bactericydal activity of granulocytes by formation in cytoplasm granules of formazan. In vial of special glass slide were added 0.025 ml of heparin (20-25 units/ml), 0.1 ml of blood, 0.05 ml of 0.15 M phosphate buffer (pH 7.2) and 0.05 ml of 0.2% solution of nitroblue tetrazolium. Vial content was mixed vigorously with pipette. Glass slide with vial was covered by filter paper, soaked with 0.85 % NaCl and another glass slide for creation of wet chamber. This construction was incubated in thermostate for 15 min at 37℃ and for 15 min at room temperature. After each stage of incubation vial content was mixed with pipette. Vial content was used for preparing cell smears on micriscope glass slides. Smears were dried, fixed with methanol and stained with 2% solution of methylene green. Evaluation of cytochemical studies was performed by semi-quantitative method using Astraldi principe based on differentiation of specific staining levels (0, +, ++, +++). Results were expressed as mean cytochemical coefficient (MCC) obtained by formula:
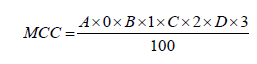
where A – number of cells with negative reaction; B – number of cells with weak positive reaction; C – number of cells with moderate positive reaction; D – number of cells with acutely positive reaction. 100 – total number of counted cells.
Statistical Analysis of Results
Statistical analysis of results was performed using Microsoft Excel 2016 data analysis. The direct quantitative data obtained from the study was used for calculation of basic statistical parameters (arithmetic mean – M, the standard deviation of the arithmetic mean – m). To evaluate the reliability of the difference between two alternative data sets, we performed Student’s t-test. The difference was considered significant under p≥0.95 (the level of significance P<0.05).
Results
OGTT revealed that organism of rats with EDM characterized by lower glucose tolerance. Glycemic curve under EDM conditions had clearer peak on 30 min, placed higher on diagram (Figure 1A) and area under glycemic curve was 2.69-fold bigger than in control group (Figure 1B). There were no significant differences in glucose tolerance between control group and control animals treated with PBM. However, treatment with PBM therapy of EDM rats improved glucose tolerance of their organism which was manifested in lower area under glycemic curve (1.83-fold than in nontreated EDM) and intermediate position of glycemic curve between control and DM group.
After investigation of glucose tolerance, we decided to evaluate efficiency of glucose uptake by rat’s blood leukocytes. We revealed that during EDM glucose uptake activity was decreased 1.18-fold. However, treatment with PBM of control animals and rats with EDM increased glucose uptake (1.21- and 1.22-fold respectively), compared to nontreated animals (Figure 2A). After that, we decided to investigate ATP content in leukocytes lysates under experimental conditions (Figure 3). We revealed that during EDM ATP content was decreased 1.39-fold comparing to control, but treatment with PBM of rats with EDM increased ATP content 1.52-fold compared to nontreated EDM animals. There were no significant changes of ATP content in control animals treated with PBM. After evaluating ATP content in leukocytes we decided to measure activity of ROS production in these cells under EDM conditions and after PBM treatment. We observed (Figure 4A), as expected, that during EDM ROS content was elevated 1.18-fold compared to control. PBM treatment of healthy animals also increased ROS content 1.15- fold but caused no significant changes of ROS content in animals with EDM. Knowing ROS content under studied conditions, we decided to measure activity of MPO. We revealed (Figure 5A), that during EDM MPO activity in blood plasma was increased 2.16- fold compared to control. PBM treatment of healthy animals also increased MPO activity 1.9-fold compared to nontreated rats. However, PBM treatment of EDM rats caused the 1.63-fold decrease of MPO activity compared to nontreated EDM animals. Contrary to these, MPO activity in leukocytes (Figure 5B) was decreased under EDM conditions and PBM treatment of healthy animals (3.17- and 2.69-fold respectively). PBM treatment of rats with EDM caused the 7.7-fold increase of MPO activity compared to nontreated EDM animals.
Figure 1: Results of OGTT: typical glycemic curves (A) and area under glycemic curves, mmol/l × h (B). In vivo study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
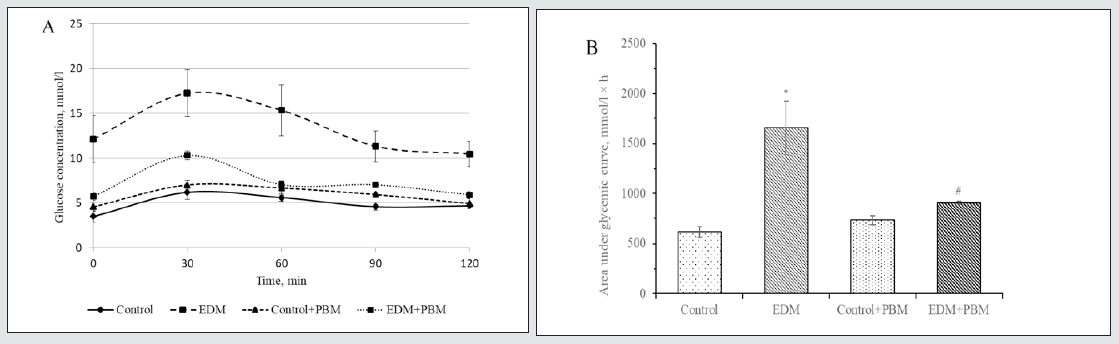
Figure 2: Fluorescence intensity of 2-NBDG in rat`s leukocytes under the studied conditions, (AU) (A); microphotographs of leukocytes fluorescence under the studied conditions, (magnification 25.2x) (B). In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
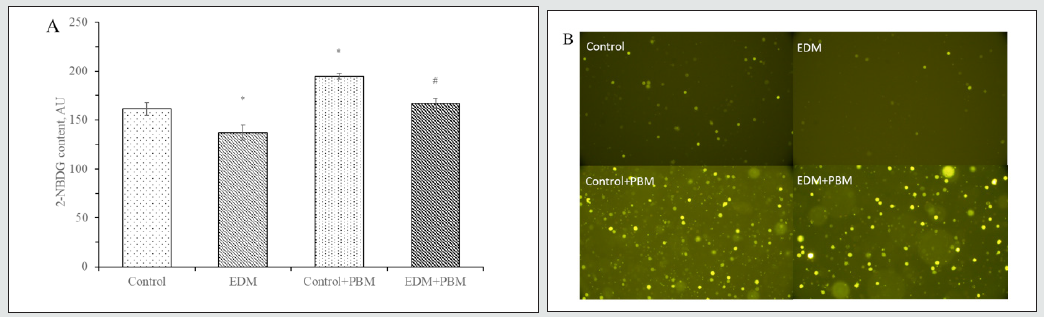
Figure 3: ATP content in leukocytes lysates, ng/μl. In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
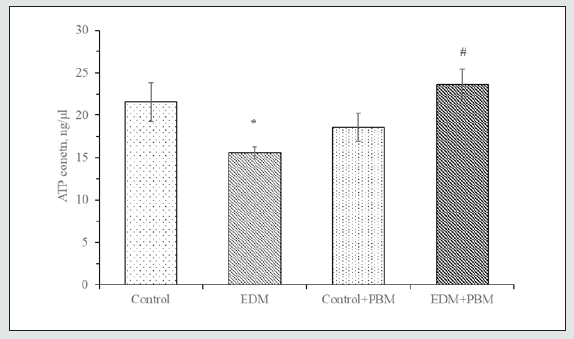
Figure 4: Fluorescence intensity of H2DCFDA in rat`s leukocytes under the studied conditions, (AU) (A); microphotographs of leukocytes fluorescence under the studied conditions, (magnification 40x) (B). In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
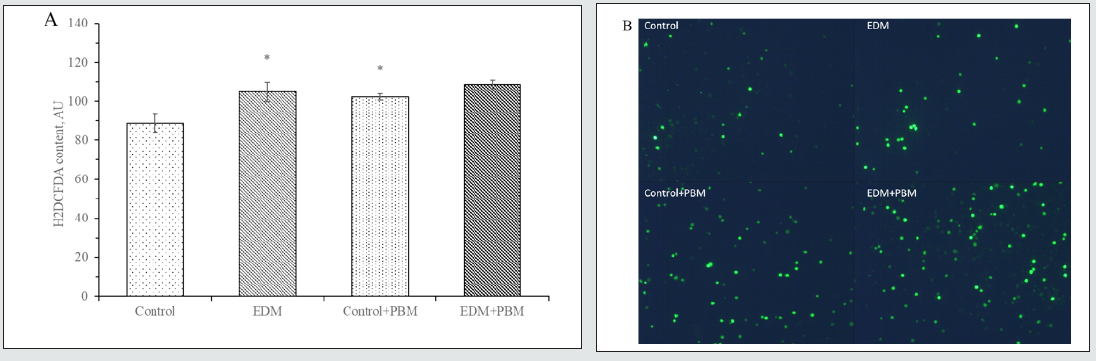
Figure 5: MPO activity in blood plasma (A) and leukocytes lysates (B), nmol H2O2/min × mg of protein. In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
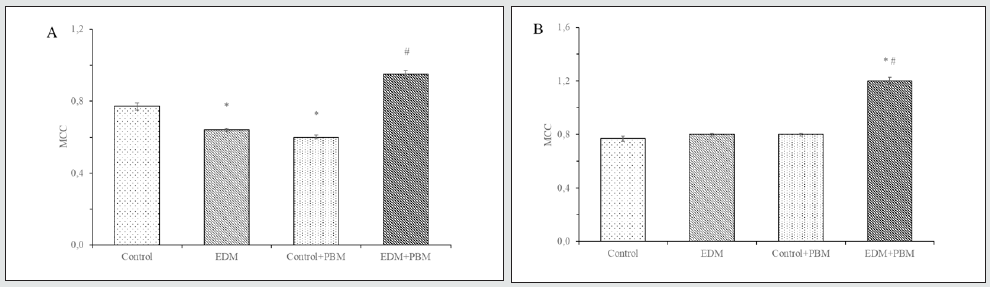
For further investigation of PBM influence on functional activity of leukocytes, we decided to evaluate changes in their phagocytic activity. We revealed, that during EDM in case of 30 min incubation of leukocytes the PI was 1.11-fold lower than in control group. PBM treatment of rats with EDM caused the 1.02-fold increase of PI after 30 min of incubation, compared to nontreated animals with EDM (Figure 6A). After 120 min of leukocytes incubation PI in rats with EDM was 1.05-fold lower compared to control group. PBM treatment of rats with EDM showed tendency to increase this index, but changes were not significant (Figure 6B). Based on these results, we calculated the index of finished phagocytosis (IFP). IFP in rats with EDM was 1.54-fold lower compared to control group (Figure 7). PBM treatment showed multidirectional changes of IFP in rats with and without EDM. In PBM-treated healthy animals IFP was 1.19-fold decreased and in PBM-treated rats with EDM IFP was 1.28- fold increased, compared to nontreated animals. After investigating phagocytic activity, we decided to evaluate bactericidal activity of neutrophil granulocytes. Measuring spontaneous production of superoxide, we observed 1.28-fold decrease of MCC during EDM (Figure 8A). PBM treatment also caused 1.20-fold decrease of MCC in healthy animals, but irradiation of rats with EDM showed 1.58- fold increase of MCC compared to nontreated animals. Considering stimulated NBT-test, we didn`t observe significant changes of MCC in control group, rats with EDM and PBM-treated healthy animals (Figure 8B). However, PBM-treated rats with EDM showed 1.5-fold increase of MCC compared to nontreated animals.
Figure 6: Phagocytic index of segmentonuclear neutrophils after 30 min (A) and 120 min (B) of incubation, %. In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
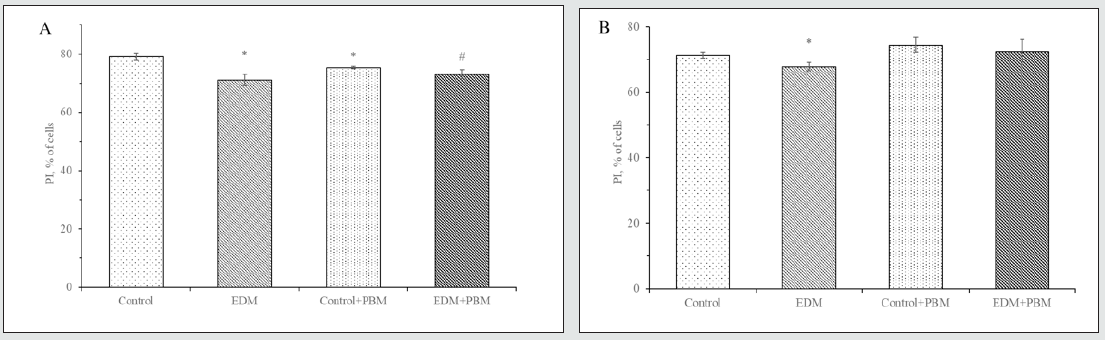
Figure 7: Changes of IFP under studied conditions. In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
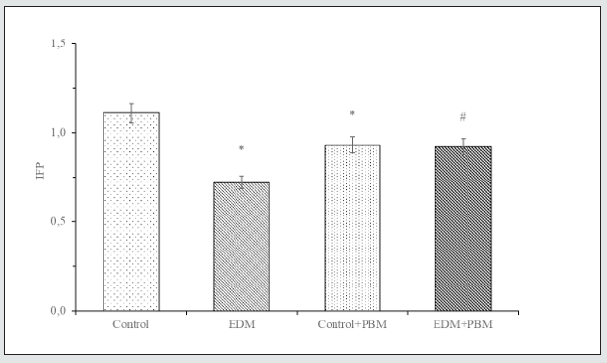
Figure 8: Spontaneous (A) and stimulated (B) NBT-test of neutrophil granulocytes. In vitro study.
* – the difference is significant compared to control, P<0.05
# – the difference is significant compared to EDM, P<0.05
Discussion
In our previous research we have demonstrated that PBM therapy are capable to lower blood glucose concentration in rats with EDM. Besides, it is more important, that PBM therapy can lower the content of glycosylated hemoglobin in EDM animals [10]. Glycosylated hemoglobin content is important index that refers to long-term changes in blood glucose concentration. Decrease of this index may indicate the prolonged hypoglycemic effect of PBM therapy, because lifetime of rat`s erythrocytes is about 50 days and 50% of glycosylated hemoglobin is formed in the last quarter of erythrocyte lifetime, which is coincides with time of our treatment [14,15]. Naturally, the question arises: how PBM therapy cause the decrease of blood glucose concentration? We presumed, that PBM therapy promotes glucose uptake by peripheral tissues. Indeed, our results of OGTT (Figure 1) are demonstrating, that hypoglycemic effect of PBM therapy can be explained by improved glucose utilization by organism. These data are consistent with the results of other researchers. In particular, it was found that light therapy promotes lowering of blood glucose concentration in patients with type 1 DM [16]. Also, in rats, PBM promotes the decrease of blood glucose level and activation of glycogen synthesis in muscles [17]. Our data and literature sources allows us to assume, that PBM therapy can influence on glucose transport in peripheral tissues. In this research we decided to concentrate on peripheral blood leukocytes because of lack of data about how glucose transport and energy supply affects their immune functions.
We found, that PBM therapy showed the improvement of glucose transport (Figure 2) as evidenced by higher fluorescence of 2-NBDG in leukocytes of irradiated healthy and diabetic rats, compared to nontreated animals. The mechanism of this action is difficult to explain as number of researches considering glucose transport and activity of its transporters in leukocytes are few and they often contradictory. In particular, it is known that in peripheral blood leukocytes present GLUT1, GLUT3, GLUT6 and GLUT9 glucose transporters. GLUT6 is low-affinity glucose transporter with unidentified primary physiological substrate and its function in leukocytes is unknown. It is suggested that GLUT6 may be involved in glucose transport between inner compartments of cell. Considering GLUT9, there are data about its presence in leukocytes but function of this transporter is also unknown. So GLUT1 and GLUT3 are considered as main glucose transporters in leukocytes [18, 9]. It is worth noting, that in rats, unlike humans, GLUT3 is expressing only in brain, so GLUT1 with highly conservative expression among mammals remains the only known target of PBM [19].
It is known that PBM therapy can activate hypoxia-inducible factor 1α (HIF-1α) in a way that, probably, do not depends from oxygen, but through activation of Mitogen-Activated Protein Kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways. HIF-1α, in turn, is capable to induce the expression of GLUT1, intensifying glucose transport into the cell [20]. Classic definition of diabetes mellitus contains interesting physiological contradiction: blood glucose concentration rises, while peripheral tissues suffer from its deficiency because of lack of insulin signaling. So we suggested, that reinforced glucose uptake by cells leads to improvement of its energy supply and functioning. To test this suggestion, we decided to identify ATP content in blood leukocytes of rats under studied conditions. There are evidences, that hyperglycemia cause the decrease of ATP content, that affects activity of antibody production in response to stimulation in B-lymphocytes [21]. We also revealed that during EDM ATP content in leukocytes decreases (Figure 3). However, PBM treatment of rats with EDM showed increased ATP content. In our opinion, this normalization of ATP content in leukocytes of treated with PBM diabetic rats is possible due to activation of oxidative phosphorylation [22] and enhanced glucose uptake by cell. These results confirm the improvement of energy supply in leukocytes by PBM therapy that can lead to normalization of their functions.
Increasing of ATP content, which is accompanied by intensification of cellular respiration should lead to increased production of ROS. Indeed, researchers revealed that PBM therapy can increase membrane potential of mitochondria and activate ROS production in mice normal cortical neurons but decrease these indices in cells with oxidative stress [23,24]. We obtained similar results for peripheral blood leukocytes. Under EDM conditions, as expected, we observed a significant increase of ROS content in leukocytes (Figure 4). Also we observed the increase of ROS production in leukocytes of healthy PBM-treated animals. During PBM treatment of rats with EDM, we didn`t observe significant changes in ROS production comparing to nontreated animals with EDM. In our previous researches [10, 25] we revealed antioxidant effect of PBM therapy. This effect was manifested as a decrease of TBA-positive products content in blood plasma, erythrocyte hemolysates and leukocyte lysates, decrease of OMP content and increase of SOD activity in PBM-treated rats with EDM. Such contradiction as increasing of ROS production and lowering of some oxidative stress indices can be explained by influence of PBM therapy on enzymatic part of pro-/antioxidant balance system, but not direct antioxidant activity of light. Because of this, we decided to investigate the effect of PBM therapy on MPO activity, as this enzyme is one of the main sources of ROS in leukocytes.
One of the main consequences of DM is the development of generalized inflammation and oxidative stress. Besides, DM patients are characterized by higher susceptibility to microbial infections and altered wound healing. These can imply the distortions in functioning of innate immune system. For example, there are defects in neutrophil chemotaxis, phagocytic and bactericidal activity in patients with diabetes. Excessive or deficient activity of MPO considered to be the main reason of these defects [26]. There is some contradiction in literature data about MPO activity during diabetes. In particular, MPO activity is decreased in leukocytes [27], but increased in blood plasma [28]. This can be explained by preactivated state of neutrophils during DM. Preactivated cells are characterized by excessive activation of NADPH oxidase and superoxide (О2-•) production which plays an important role in bactericidal function of neutrophils, but also can lead to development of oxidative stress and apoptosis if produced in plenty [29]. While such preactivation, negatively affects antimicrobial activity of neutrophils, it also takes part in development of inflammation through activation of neutrophils to release MPO in blood plasma due to action of pro-inflammatory cytokines (in particular TNF-α) [30, 31].
As a result of our research, we found the decreased activity of MPO in leukocytes of rats with EDM. This decrease of MPO activity can be explained by lower content of Advanced Glycosylation End Products (AGEs) in leukocytes during DM. There are data, that AGEs can stimulate MPO production through interaction with RAGE [32]. Besides, such decrease of MPO activity in leukocytes during EDM can partially explain higher ROS content in leukocytes because lack of MPO and catalase activity prevents to utilize hydrogen peroxide [33]. We revealed the increase of MPO activity in blood plasma of rats with EDM, that confirms involvement of this enzyme in development of oxidative stress and inflammation. However, PBM treatment of EDM rats revealed the increase of MPO activity in leukocytes and decrease in blood plasma. We think this may indicate the improvement of leukocyte`s functional activity and lowering of oxidative stress in rats with EDM under PBM treatment.
For further investigation of PBM therapy effect on functional activity of leukocytes, we decided to examine phagocytic and bactericidal activity of leukocytes. Researches about activity of immune response in patients with DM first emerged in 1960-s. In particular, there were shown that chemotaxis of Polymorphonuclear Leukocytes (PMNL) was distorted during ketoacidosis. Aleksievich et al, revealed impaired phagocytosis in leukocytes of DM patients and this was related to glycemic control, where better control of glucose level during 3-month period improved phagocytosis activity [34]. It is also known, that decrease of MPO activity leads to suppressing of phagocytosis in neutrophils and increases the risk of infections in DM patients [35]. In general, there is the whole spectrum of defects in work of immune system during DM. However, the main factor that promotes increased susceptibility to infections is deterioration of leukocyte function [36]. In our research we also revealed the decrease of phagocytosis activity in leukocytes of rats with EDM. This expresses as the decrease of PI in segmentonuclear neutrophils after 30 min of incubation and in the decrease of IFP. In PBM-treated rats with EDM we find the increase of phagocytosis activity compared to nontreated animals with EDM. Phagocytosis is a complex energy-dependent process that requires precise reorganization of cytoskeleton and neutrophil degranulation. Besides, there are researches, indicating that phagocytosis process in leukocytes depends from energy supply through breakdown of glucose by glycolysis [37]. Changes in glycolysis under diabetic conditions in leukocytes are scarcely studied, but some works indicating that glycolysis is downregulated due to decrease of phosphofructokinase activity [38]. Other researchers, vice versa, revealed increased activity of phosphofructokinase, but decreased activity of glucose-6-phosphate dehydrogenase – key enzyme of pentose phosphate pathway. Thus, there is no doubt that during DM carbohydrate metabolism in leukocytes is distorted. Also, it is important to note, that insulin therapy can neutralize the negative effect of DM on carbohydrate metabolism in leukocytes even if there are no changes in hyperglycemia [39]. This indicates that the disturbance in glucose uptake is responsible for impaired functions of leukocytes during DM.
Besides phagocytic activity, we determined bactericidal ability of leukocytes by performing NBT-test which indicates NADPH oxidase activity and superoxide anion production in neutrophils. We revealed, that during EDM there is decrease of spontaneous О2-• production but there are no significant changes in stimulated О2-• production. However, PBM therapy treatment revealed significant increase of spontaneous and stimulated О2-• production. There are contradictive data in literature about ROS production in leukocytes during DM. Some researchers report about increased ROS production in neutrophils due to development of oxidative stress during DM. This is also confirmed by our data (Figure 4A). At the same time, another researches indicating the suppressing of respiratory burst during DM due to decreased activity of NADPH oxidase. These occurs because of hyperglycemia-induced inhibition of glucose-6-phosphate dehydrogenase activity, one of the main function of which is the supporting the level of NADPH, substrate for NADPH oxidase [40]. This is also consistent with our data of NBT-test, that demonstrates the decrease of О2-• production during EDM and the increase after PBM therapy treatment. Thus, antioxidant activity and antihyperglycemic effect of PBM therapy along with facilitation of glucose uptake by leukocytes, evidences about its positive influence on energy supply and pro-/antioxidant balance of cells. All these afterwards lead to improvement of functional activity of leukocytes.
There are limitations in this study that should be noted. The method of leukocytes extraction allows us to evaluate energy supply, ROS content and MPO activity only in the whole WBC population, but not in subpopulations. Further research with separated subpopulations of WBC is needed. Biological effects of PBM therapy may be dependent on the dosimetry characteristics, yet we only used one type of LED matrices for all studies, thus other spectrum or energy densities may have different action. Loss of function studies in vitro (using ATP and ROS neutralization agents) and time-course kinetic study with H2DCFDA would further enhance the specificity of our observations.
Conclusions
PBM therapy treatment of rats with EDM causes improvement of glucose tolerance, activation of glucose uptake and ATP production in leukocytes that accompanied by increased ROS production. Besides, we revealed that PBM therapy treatment causes increase of MPO activity, phagocytic activity and superoxide anion production in leukocytes. The decrease of oxidative stress indices, that was revealed earlier, together with observed in this research increase of ROS content and activity of superoxide anion production, evidence about positive effect of PBM therapy on pro-/ antioxidant balance in leukocytes. This balance is very important and, as we think, processes of ROS accumulation in the cell during oxidative stress, that accompanies DM, and specific ROS production in leukocytes for performing their function as immunocompetent cells should be clearly distinguished. We believe, that found in this study positive effects of PBM therapy during EDM on functional activity of leukocytes are caused, in particular, by improvement of their energy supply due to higher glucose uptake in cell and increased production of ATP.
Acknowledgements
We thank to Senior Researcher Roman V. Gamernyk from Department of Experimental Physics of Ivan Franko National University of Lviv for helping in measurements of dosimetry data. We are also grateful to Senior Researcher Manko B.O. from Interuniversity Collective Center of Cell Biology and Bioenergy of Ivan Franko National University of Lviv for help and providing the equipment for microscopy experiments.
Supporting information
Ethics Approval and Consent to Participate
Manipulations with animals were conducted according to General Ethical Principles of Animal Experiments approved by the First National Congress for Bioethics (Kyiv, 2001), which agrees with the provisions of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986). Approval was taken from ethical committee of Ivan Franko National University of Lviv.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare that they have no competing interests
Funding
Not applicable
Authors’ contributions
This study was designed, directed and coordinated by NS; ML acted as the principal investigator, provided conceptual and technical guidance for all aspects of the project. OK and NY planned and performed experiments and analyzed data. The manuscript was written by OK, ML and NY and commented on by NS. All authors read and approved the final manuscript.
References
- Stringer DM, Zahradka P, Taylor CG (2015) Glucose transporters: Cellular links to hyperglycemia in insulin resistance and diabetes. Nutr Rev 73(3): 140-154.
- Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93(3): 993-1017.
- Bryant NJ, Govers R, James DE (2002) Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3(4): 267-277.
- Fazakerley DJ, Krycer JR, Kearney AL, Hocking SL, James DE (2019) Muscle and adipose tissue insulin resistance: malady without mechanism?. J Lipid Res 60(10): 1720-1732.
- Luni C, Marth JD, Doyle FJ 3rd. (2012) Computational modeling of glucose transport in pancreatic β-cells identifies metabolic thresholds and therapeutic targets in diabetes. PLoS One 7(12): e53130.
- Lau EYM, Carroll EC, Callender LA, Hood GA, Berryman V, et al. (2019) Type 2 diabetes is associated with the accumulation of senescent T cells. Clin Exp Immunol 197(2): 205-213.
- Soongsathitanon J, Umsa-Ard W, Thongboonkerd V (2019) Proteomic analysis of peripheral blood polymorphonuclear cells (PBMCs) reveals alteration of neutrophil extracellular trap (NET) components in uncontrolled diabetes. Mol Cell Biochem 461(1-2): 1-14.
- Otton R, Carvalho CR, Mendonça JR, Curi R (2002) Low proliferation capacity of lymphocytes from alloxan-diabetic rats: Involvement of high glucose and tyrosine phosphorylation of Shc and IRS-1. Life Sci 71(23): 2759-2771.
- Piatkiewicz P, Czech A, Tatoń J (2007) Glucose transport in human peripheral blood lymphocytes influenced by type 2 diabetes mellitus. Arch Immunol Ther Exp (Warsz) 55(2): 119-126.
- Karmash OI, Lіuta MY, Yefimenko NV, Korobov AM, Sybirna NO (2018) The influence of low-level light radiation of red spectrum diapason on glycemic profile and physicochemical characteristics of rat’s erythrocytes in diabetes mellitus. Fiziol Zh 64(6): 68-76.
- Sakaguchi K, Takeda K, Maeda M, Ogawa W, Sato T, et al. (2015) Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int 7(1): 53-58.
- Stoika R, Kashchak N, Lutsik Kordovsky M, Boyko M, Tsyrulnyk A, et al. (2001) In vitro response of phagocytic cells to immunomodulating agents. Med Sci Monit 7(4): 652-658.
- Bazhora YI (1981) Simplified method of NBT-test. Lab delo 4: 198-200.
- Ivanov IT (2007) Allometric dependence of the life span of mammal erythrocytes on thermal stability and sphingomyelin content of plasma membranes. Comp Biochem Physiol A Mol Integr Physiol 147(4): 876-884
- Warner EA, Herold AH (2011) Interpreting Laboratory Tests. In: Rakel RE, Rakel DP, editors. Textbook of Family Medicine. Philadelphia: Saunders, 176-204.
- Longo L, Postiglione M, Buccioni T, Longo D (2009) The effects of Low Level LASER Therapy (LLLT) on blood glucose levels in patients with Diabetes Mellitus type I: A case report. AIP Conference Proceedings 1142: 92-95.
- Castro KMR, de Paiva Carvalho RL, Junior GMR, Tavares BA, Simionato LH, et al. (2020) Can photobiomodulation therapy (PBMT) control blood glucose levels and alter muscle glycogen synthesis?. J Photochem Photobiol B 207: 111877.
- Vrhovac V, Breljak D, Sabolić I (2014) Glucose transporters in the mammalian blood cells. Periodicum Biologorum 116(2): 131-138.
- Simmons RA (2017) 43 - Cell Glucose Transport and Glucose Handling During Fetal and Neonatal Development. Fetal and Neonatal Physiology (Fifth Edition). Elsevier 428-435.
- Hamblin MR (2018) Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol 94(2): 199-212.
- Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Maciejewska I, Szutowicz A, et al. (2013) High glucose concentration impairs ATP outflow and immunoglobulin production by human peripheral B lymphocytes: Involvement of P2X7 receptor. Immunobiology 218(4): 591-601.
- Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8): 607-610.
- Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR (2013) Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics 6(10): 829-838.
- Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4(3): 337-361.
- Karmash O, Liuta M, Korobov A, Sybirna N (2020) Effect of Photomodulation Therapy on Development of Oxidative Stress in Blood Leukocytes of Rats with Streptozocin-Induced Diabetes Mellitus. Cytology and Genetics 54: 456-464.
- Ferdous M, R SC, Mudi SR, Ali M, Jasmin S, et al. (2020) Expression of neutrophil elastase and myeloperoxidase mRNA in patients with newly diagnosed type 2 diabetes mellitus. Diabetes Metab Syndr 14(2): 83-85.
- Unubol M, Yavasoglu I, Kacar F, Guney E, Omurlu IK, et al. (2015) Relationship between glycemic control and histochemical myeloperoxidase activity in neutrophils in patients with type 2 diabetes. Diabetol Metab Syndr 7: 119
- Gómez García A, Rivera Rodríguez M, Gómez Alonso C, Rodríguez Ochoa DY, Alvarez Aguilar C (2015) Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab J 39(1): 59-65.
- Dinçer Y, Akçay T, Ilkova H, Alademir Z, Ozbay G (2003) DNA damage and antioxidant defense in peripheral leukocytes of patients with Type I diabetes mellitus. Mutat Res 527(1-2): 49-55.
- Bosco AM, Almeida BFM, Valadares TC, Baptistiolli L, Hoffmann DJ, et al. (2018) Preactivation of neutrophils and systemic oxidative stress in dogs with hyperleptinemia. Vet Immunol Immunopathol 202: 18-24.
- Nauseef WM (2001) Contributions of myeloperoxidase to proinflammatory events: More than an antimicrobial system. Int J Hematol 74(2): 125-133.
- Lu H, Xu S, Liang X, Dai Y, Huang Z, et al. (2019) Advanced Glycated End Products Alter Neutrophil Effect on Regulation of CD4+ T Cell Differentiation Through Induction of Myeloperoxidase and Neutrophil Elastase Activities. Inflammation 42(2): 559-571.
- Zozulińska DA, Wierusz-Wysocka B, Wysocki H, Majchrzak AE, Wykretowicz A (1996) The influence of insulin-dependent diabetes mellitus (IDDM) duration on superoxide anion and hydrogen peroxide production by polymorphonuclear neutrophils. Diabetes Res Clin Pract 33(3): 139-144.
- Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG (1995) Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: Abnormalities in metabolism and function. Ann Intern Med 123(12): 919-924.
- Alba Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, at al. (2007) Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res 40(8): 1037-1044.
- Dulkadiroğlu E, Özden H, Demİrcİ H (2021) The evaluation of intracellular energy metabolism in prediabetic patients and patients newly diagnosed with type 2 diabetes mellitus. Turk J Med Sci 51(1): 238-245.
- Kumar S, Dikshit M (2019) Metabolic Insight of Neutrophils in Health and Disease. Front Immunol 10: 2099.
- Esmann V (1983) The polymorphonuclear leukocyte in diabetes mellitus. J Clin Chem Clin Biochem 21(9): 561-567.
- Alba-Loureiro TC, Hirabara SM, Mendonça JR, Curi R, Pithon-Curi TC (2006) Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol 188(2): 295-303.
- Huang J, Xiao Y, Xu A, Zhou Z (2016) Neutrophils in type 1 diabetes. J Diabetes Investig 7(5): 652-663.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




