Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
Sudanese Women has a High Frequency of Risk Factors for Gestational Diabetes Volume 3 - Issue 4
Nahla Ahmed Mohamed Abdurahman1*, Mohamed Ibrahim Asadalla Murad2, Mohamed Abbas Ahmed Khalafallah2, Mildred Francis Amule2, Mohamed Ahmed Ibrahim Ahmed3 and Nassreldeen Khalid Abdurahman Adam4
- 1Department of Biochemistry, Assistant professor of Biochemistry, Faculty of Medicine, Nile Valley University, Sudan
- 2Faculty of Medicine, Sudan International University, Sudan
- 3Department of Microbiology, Assistant professor of Microbiology, Faculty of Medicine, Nile Valley University, Sudan
- 4Assistant professor of hematology, Faculty of Medical Laboratory Science, University of Al Fashir, Sudan
Received:June 10, 2021; Published:July 7, 2021
Corresponding author: Dr. Nahla Ahmed Mohammed Abdurrahman, Department of Biochemistry, Assistant professor of Biochemistry, Nile Valley University, Faculty of Medicine, Atbara, Sudan
DOI: 10.32474/ADO.2021.03.000169
Abstract
Gestational diabetes (GD)is the most prevalent metabolic condition associated with pregnancy, and it has a number of negative consequences for both the mother and the child. It’s also one of the easiest diseases to manage throughout pregnancy. A variety of risk factors have been identified and confirmed to have a significant relationship with the disease. These risk factors are quite straightforward to detect, and screening people who are at risk might help to reduce the number of problems connected with GD. In the long run, it would be more cost effective for the woman and child, their family, and the community. This study aims to emphasize these risk variables and give an estimate of the number of patients with GD who will be admitted to the hospital. Diagnosing women with GD allows them to be educated, treated, and have a better long-term prognosis.
Objective: Investigate risk factors for GD, estimate prevalence of GD, explore effects of DM on pregnancy (miscarriage, Intrauterine Fetal Death (IUFD), Intrauterine Growth Restriction (IUGR), Urinary tract infection (UTI), polyhydramnios), and identify neonatal complication of GD in pregnant women at AL turkey hospital.
Materials and Methods: From November 2018 to May 2019, a hospital-based study was conducted in pregnant women who came in for follow-up and admitted patients at Al Turkey Hospital. The goal of the study was to identify risk variables and estimate the prevalence of GD. A questionnaire was filled out that included personal information besides anthropometric measurements and clinical historical information.
Statistical Analysis: The Statistical Package for Social Sciences (SPSS) SPSS version 20 was used. Chi- Squire test was used to count the distribution of study participants in the study variables.
Results: The majority of women who participate in this study were housewives accounted for 68 % from and 61 % were from Urban areas. Age distribution was revealed that the most age group was found to be (26-35) years 78 % . Gestational age 42 % with 35- 40 weeks followed by 41 % with 24 -34 weeks and only >24 weeks 15 % and least number 2 % more than 40 gestational age. BMI majority 57% were in normal weight 18-24 kg/m2. Parity 60% delivered From 3-6 times and 75% of them deliver by normal vaginal delivery. 2% of baby weight more than 4.5 kg (macrosomic) and 3% with congenital anomalies. Babies delivered with neonatal jaundice were 5% and 4% delivered baby with hypoglycemia. Conclusion: 78% of women in the ages of 26- 35. The most relevant risk variables in our study to GD were, obesity7%, family history of GD 5%, congenital abnormalities Neonatal jaundice5%, hypoglycemia 4%, past history of GD 3%, multiparty3%, and mother with history of fetal difficulties macrocosmic infant 2% did not affect presence of GD. Women with GD may have a secondary cause of illness, such as metabolic syndrome or a hereditary predisposition to diabetes
Keywords: Gestational diabetes mellitus; Khartoum; Sudan
Abbreviations: GDM: Gestational Diabetes Mellitus; IGT: Impaired Glucose Tolerance; BMI: Body Mass Index; PIH: Pregnancy Induce Hypertension; C-section: Cesarean Section
Introduction
Gestational diabetes mellitus GDM is defined as any degree of glucose intolerance (impairment of glucose metabolism) that occurs during pregnancy or is first recognized during pregnancy [1,2]. It has a strong link to the development of a range of deleterious pregnancy outcomes, contributing considerably to maternal, perinatal morbidity and death, and can have long-term effects for both mother and offspring’s health [3]. Complications for mothers include the risk of impaired glucose tolerance (IGT) and type 2 diabetes in the years after delivery, as well as obesity and the development of IGT and diabetes in early adulthood for kids [4]. Nearly 90% of all DM-complicated pregnancies are caused by GDM [5]. GDM is becoming more common around the world, posing a major public health threat [6,7]. This rate varies by setting and population, with a prevalence ranging from 1–14 % depending on the population studied and the diagnostic tests used [8]. The frequency of GDM in Africa varies greatly, from a low %age in Tanzania to 13.9 % in Nigeria. However, according to a recent comprehensive assessment of GDM in Sub-Saharan Africa, data on the burden of GDM in various African nations, including Sudan, is lacking [9]. GDM has been linked to a number of characteristics, including age, parity, education, a family history of diabetes, and obesity [10]. In Sudan, no data on GDM has been released. For practicing doctors, caregivers, and health planners, as well as academics, determining the prevalence and risk factors for GDM is critical. The purpose of this study was to look at the prevalence and risk factors for GDM in Sudan.
Materials and Method
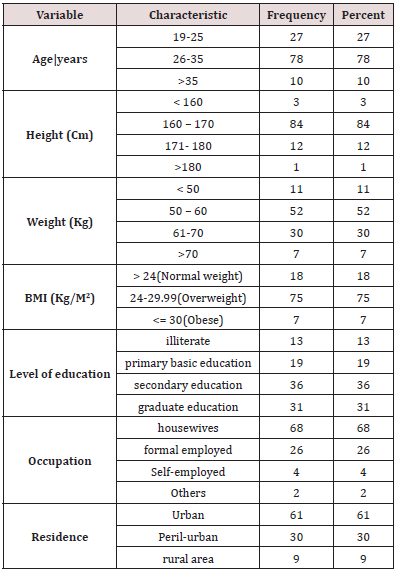
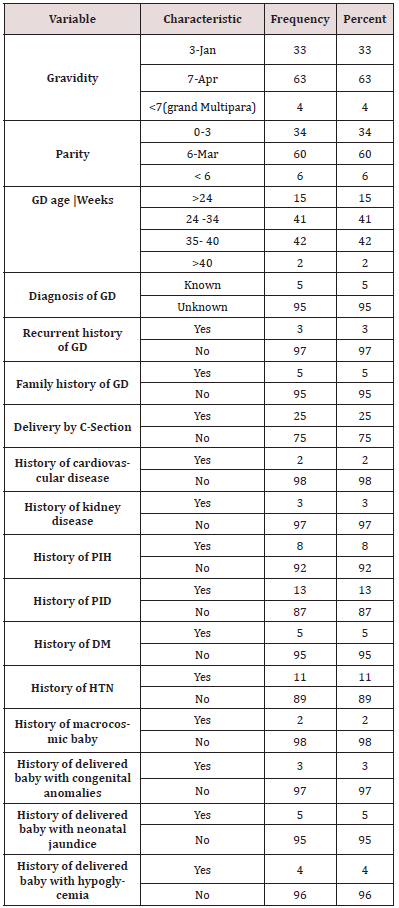
Study Design, Area, Period& Population From November 2018 to May 2019, a hospital-based research was conducted in GD pregnant women who came in for follow-up and admitted patients at Al Turkey Hospital from varied ages, tribes, homes, vocations, and socioeconomic status. Using a questionnaire for personal information and anthropometric measurements (weight, height, and then calculation of BMI using the formula BMI= (weight in kg)/(height in m)2 [11], the review looked at 100 women to identify risk factors and estimate the prevalence of GD. Clinical historical information include (history of macrocosmic baby, history of pregnancy induce hypertension PIH, family history of GD, diagnosis of GD, delivered baby with history of hypoglycemia etc….(Table 1).
Statistical Analysis
The Statistical Package for Social Sciences software (SPSS, version 20.00) used for data processing and statistical analysis Data collectors training lasted in two days.
Ethical Consideration
Permission obtained from the manager of AL turkey hospital verbal consent obtained from those who participated in the study.
Results
The most common age group was (26-35) years, accounting for 78 percent of the total. The studied population appears to have a higher level of education, with those with a secondary education accounting for 36% of the total. Housewives accounted for 68 percent of the women examined, with a moderate income and the majority live in Urban 61% (Table 1). The BMI of 75% of the women was 18-24 kg/m2, suggesting that they had a normal BMI. In terms of obstetric history, 63 % of the women interviewed fell within the Gravidity 4-7 range. Parity 60% delivered From 3-6 times. With 35- 40 weeks, gestational age is 42 percent. Delivery method Seventyfive percent of the moms polled said they had a normal vaginal birth. 2 % of moms delivered babies weighing more than 4.5 kg (macrosomic), and 3 % of babies had congenital abnormalities. The percentage of babies born with neonatal jaundice was 5%, while the percentage of babies born with hypoglycemia was 4%. Medical and historical of women showed that: of study population diagnosed with GD, Cardiovascular disease, Kidney disease, Pregnancy induce hypertension, Pelvic inflammatory diseases, DM and HTN (5 , 2, 3, 8, 13, 5 and 11 % ) respectively (Table 2).
BMI= Body Mass Index; Cm=centimeter, Kg=kilogram, M=meter
GD= Gestational Diabetes; C- Section=Cesarean Section; PIH= History of pregnancy induce hypertension; PID= History of pelvic inflammatory disease; HTN=Hypertension; DM= Diabetes mellitus
Discussion
In this study, diabetic women had a low prevalence of age, parity, place of residence, education, and a high BMI, indicating a low risk of GD. These findings matched those of Rayis, DA et al, [12] who did research at Sudan’s Saad Abuelela Maternity Hospital and the same results were found in Ghanaian women [10,12]. On other way, history of macrosomia (fetal macrosomia is a term used to define newborns who are significantly larger than average (birth weight ≥4,000 g)) was found in 2 fetal of study population indicating that macrosomia has low risk in GDM, because between 15 and 45% of newborns of mothers with GD mellitus are macrosomic (in comparison to 12% of newborns of normal mothers) and that maternal obesity has a strong and independent effect on fetal macrosomia which was observed in only 7% of studied women [13]. This finding was in agreement with that of Yogev Y, and Langer O, et al, [14] which found that the comparison of obese women to normal-weight women, the newborns of obese women had more than double the risk of macrosomia compared to those of women with normal weight. Yogev Y, and Langer O, 2008 [14] also found that Gestational age at delivery, maternal, pregnancy weight gain, maternal height, hypertension and cigarette smoking also have a significant impact [14].
In a prospective trial of around 500 women divided into two groups, 130 were diagnosed with GDM while the other 370 had a normal pregnancy. In this study, [15] found that maternal age, family history of DM, and GDM history were the most relevant risk factors for GDM diagnosis, as evidenced in a small number of our research participants [15]. The small size of the study sample, which included one hundred participants from pregnant women, may be the main factor in the large discrepancy with similar studies and may constitute the most important determinants of the study.
Conclusion
The majority of women (78%) were between the ages of 26- 35. The most relevant risk variables in our study to GD were, obesity7%, family history of GD 5%, congenital abnormalities Neonatal jaundice5%, hypoglycemia 4%, past history of GD 3%, multiparty3%, and mother with history of fetal difficulties macrocosmic infant 2% did not affect presence of GD. Women with GD may have a secondary cause of illness, such as metabolic syndrome or a hereditary predisposition to diabetes.
Disclosure of Interest
The authors declare that they have no known competing financial interests or personal relationships and conflicts of interest that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank all obstetricians of the Al Turkey Hospital and pregnant ladies who participated in the study and contributed to the collection of data.
Recommendation
1. Conducting more studies on GD and maternal morbidity and death.
2. Initiating development efforts to better socioeconomic conditions.
3. Mothers’ education and counseling programs on the importance of family planning and risk factors for GD.
4. Advise pregnant women, particularly diabetes mothers, about the need of proper prenatal treatment and follow-up.
References
- Metzger BE (1991) Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes 40(Suppl 2):197-201
- Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, et al. (2007) Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 30(Suppl 2): 251-260.
- Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, et al. (2015) The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 131(Suppl 3): 173-211.
- American Diabetes Association (2004) Gestational diabetes mellitus. Diabetes Care 27(Suppl 1): 88-90.
- Gestational diabetes (1995) National Diabetes Data Group (U.S.), National Institute of Diabetes and Digestive and Kidney Diseases (U.S.) and National Institutes of Health (U.S.).
- Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM (2004) An increase in the incidence of gestational diabetes mellitus: Northern California 1991-2000. Obstet Gynecol 103: 526-533.
- Dabelea D, Snell Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, et al. (2005) Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 28(3): 579-84.
- Kim C, Newton KM, Knopp RH (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25(10): 1862-1868.
- Macaulay S, Ngobeni M, Dunger DB, Norris SA (2018) The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract 139: 278-287.
- Olagbuji BN, Atiba AS, Olofinbiyi BA, Akintayo AA, Awoleke JO, et al. (2015) Prevalence of and risk factors for gestational diabetes using 1999, 2013 WHO and IADPSG criteria upon implementation of a universal one-step screening and diagnostic strategy in a sub-Saharan 388 D. A. RAYIS ET AL. African population. European Journal of Obstetrics & Gynecology and Reproductive Biology 189: 27-32.
- Ng M, Fleming T, Robinson M, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384(9945): 766-81.
- Rayis DA, Musa IR, Al Shafei AI, Moheldein AH, El Gendy OA, et al. (2021) High haemoglobin levels in early pregnancy and gestational diabetes mellitus among Sudanese women. J Obstet Gynaecol 41(3): 385-389.
- Ehrenberg HM, Mercer BM, Catalano PM (2004) The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol 191: 964-968.
- Yogev Y, Langer O (2008) Pregnancy outcome in obese and morbidly obese gestational diabetic women. Eur J Obstet Gynecol Reprod Biol 137(1): 21-26.
- Laily Najafi (2020) Risk factors in gestational diabetes mellitus. Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran. Immunological Disorders and Immunotherapy 5(2).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...