
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Review Article(ISSN: 2638-5910) 
Potential Management of Gestational Diabetes and its complications Using Antidiabetic Medicinal Plants of Cameroon: Case Study of Deficiency in Hormones Production, Uterine and Placental Abnormalities and Glucose Teratogenic Capability Volume 3 - Issue 3
Tsabang Nolé1* and Tsambang Djeufack Wilfried Lionel2
- 1Higher Institute of Environmental Sciences, Cameroon
- 2Centre de Cardiologie et Medical, Yaounde, Cameroon
Received:April 30, 2021; Published: May 07, 2021
Corresponding author: Tsabang Nolé, Higher Institute of Environmental Sciences, Yaounde, Cameroon
DOI: 10.32474/ADO.2021.03.000165
Abstract
The prevalence of Gestational Diabetes Mellitus (GDM) is raising worldwide parallel to the increment in the prevalence of obesity and Type2 Diabetes Mellitus. Gestational diabetes mellitus (GDM) affects about 6% of gestations and manifests after the 24th week of gestation. GDM is associated with motherly and neonatal adverse consequences. These consequences are not known by the populations of neighboring areas of developing Countries like Cameroon. Maintaining adequate blood glucose levels in GDM reduces morbidity for both mother and baby. For the first time a research on antidiabetic plants commonly used in Cameroon is done to select amongst them those which can help in the regulation of deficiency in hormones production, in the improvement of uterine and placental abnormalities and glucose teratogenic capability. To achieve this objective a systematical search was done using engines related to the effects such as: Enhance a drop-in insulin performance in its appropriate receptors in gestational diabetes; regulate hormones secreted by the placenta and fetus; prevent macrosomia; prevent growth disturbances; reduce congenital and maternal anomalies in gestational diabetes like (preeclampsia; hypotension; vascular lesions and post-gestational diabetes). A variety of placental hormones including estrogens, cortisol, human chorionic go-nadotrophin (HCG), progesterone and human placental lactogen are produced to maintain the pregnancy, and the risk of insulin resistance becomes greater. Some of these hormones can have a blocking effect on insulin, called contra-insulin effect, which usually begins about 20 to 24 weeks into the pregnancy. Typically, the pancreas is able to make additional insulin to overcome insulin resistance, but when the production of insulin is not enough to overcome the effect of the placental hormones, gestational diabetes results. Gestational diabetes is not caused by a lack of insulin, but by other hormones produced during pregnancy that can make insulin less effective, a condition referred to as insulin resistance. According to the recent redefinition, GDM is characterized by insulin resistance and fasting glycaemia levels higher than 92 mg per ml. Do known anti-diabetic plants regularly used in Cameroon help in the management of hormones deficiency production, uterine and placental abnormalities and glucose teratogenic capability connected to this disease? Zingiber officinale Lin. is currently the most potent herbal medicine habitually used to control the blood glucose levels and help prevent disorders resulting to the consequences of severe maternal hyperglycemia on postnatal development of offspring.
Keywords:Keywords: Gestational Diabetes; Hormonal Deficiency; Intrauterine Growth Restriction; Macrosomia; Microsomia; Hypoglycemia; Fetal Hyperinsulinemia; Glucose Teratogenic Capability
Abbreviations: GDM: Gestational diabetes mellitus; ESC: Embryonic stem cells; T2GDM: Type2 diabetes mellitus; HCG: Human chorionic gonadotropin; LH: ; FSH: ; CAMP: Cyclic adenosine monophosphate; PKA: Protein kinase; HPL: Human Placental Lactogen; IGF-1: insulin-like growth factor-1; PI3K: Phosphoinositide 3-kinase; ERK: Extracellular signal-regulated kinases; VEGF : Vascular endothelial growth factor; HUVEC: Human umbilical vein endothelial cells; VEGFR-1: Vascular endothelial growth factor receptor 1; VEGFR 2: Vascular endothelial growth factor receptor 2; MMP-1: Matrix metalloproteinase-1; MMP-2: Matrix meitalloproteinase-2; FGF21: Fibroblast growth factor 21; EVT: Endovascular Treatment; PAPP-A: Pregnancy-associated plasma protein A; IGFBP-4: Insulin-like growth factor-binding protein 4; IUP: intrauterine pregnancy; FBS: Fasting blood sugar; AMPk : Activated protein kinase: AGA: Appropriate for gestational age; NPY: Neuropeptide; UCP1: Uncoupling Protein 1; Pdx1: Pancreatic and Duodenal Home box 1;Gch1: GTPcyclohydrolase 1; PDX1: Pancreatic and duodenal home box 1; IUGR: Intrauterine growth retardation; Fgfr1: Fibroblast growth factor receptor 1; CaMKK: Calmodulin dependent kinase kinase; Thr172by AMPK: Threonine 172 Thr172) 72 by activated protein kinase; AKT1: Protein kinase B; KATP Channel: ATP-sensitive potassium channel; DCM : DNA Control Medicine ; StAR: Steroidogenic acute regulatory; LHR: Luteinizing hormone receptor; AR: Androgen receptor; PCOS: Polycystic ovary syndrome; FBS: Fasting blood sugar; DPPH: Diphenyl-2-picrylhydrazyl; NO: Nitric oxide; AGI: Alpha glucosidase inhibitors; DPP-4dipeptidyl peptidase-4 inhibitors; PE: Pulmonary embolism; Kp-10: kisspeptin-10; IGFBP-4: Insulin-like growth factor-binding protein 4; IGF- 1 and 2: Insulin-like growth factor-1 and 2 ; ROC: Receiver-operating characteristic
Introduction
Gestational Diabetes Mellitus (GDM) is a condition in which a hormone produced by the placenta inhibits the body from using insulin successfully. Glucose builds up in the blood instead of being engrossed by the cells. The question is what hormones does the placenta produce which cause diabetes during pregnancy? Pregnancy is a period of changes in hormone levels that can have many effects. As the placenta grows or as the pregnancy advances, there are six key hormones which play important roles in baby’s development, and other effects [1, 2].
Human Chorionic Gonadotropin (HCG)
HCG is the most an important hormone in early pregnancy, produced by the placenta after implantation, and supports the function of a temporary structure in the ovaries, called corpus luteum which produces other key hormones during early pregnancy. It belongs to the glycoprotein family hormone, sharing similarities with other members of this family, such as hypophysary LH and FSH. Different in-vitro studies have also reported that HCG is important for immunotolerance, as it overpowers the maternal immunological system [2-5].
Progesterone
Progesterone is a steroid hormone, crucial for gestational maintenance, produced by the corpus luteum, owing to HCG stimulation, during the first weeks of pregnancy. After 6–8 weeks of gestation until the end of pregnancy, as HCG concentration declines, the placenta gradually becomes the main source of progesterone, owing to the formation of the syncytial layer. Progesterone exercises genomic and non-genomic actions [2-5]
Progesterone Genomic
As the main functions progesterone stimulates in the uterus in-vitro decidualization of human ESC, by increasing CAMP levels and activating PKA signalling pathway. This steroid also promotes embryo implantation in animal models, by blocking oestrogens pro-proliferative effect in uterine epithelial cells and inducing genes that promote uterine receptivity [2-5].
Non-genomic actions
In addition to placentation, progesterone stimulates the growth of blood vessels that supply the lining of the uterus. It also stimulates the lining to release nutrients, providing nourishment for the early embryo. In addition, progesterone inhibits the contraction of smooth muscle in the uterus so that it grows like the baby. It promotes the growth of breast tissue and the development of milk ducts. Progesterone prevents lactations during pregnancy, which does not begin until levels drop after birth. This hormone also plays an important role in preparing for childbirth: it strengthens the muscles of the pelvic wall necessary for labor [2-5].
Estrogens
As with progesterone, the corpus luteum produces estrogen in the early stages of pregnancy before the placenta takes over. Estrogen is actually a collective group of similar compounds: estrone, 17β-estradiol most abundant, estriol, and andoestetrol. Oestrogen promotes embryo implantation, as it stimulates endometrial growth and differentiation. In human endometrial explant cultures, oestrogen modulates the expression of several genes that participate in endometrial maturation and differentiation. Estrogen helps the uterus to grow and maintain its lining. It supports the development of the fetus, including the development of organs and body systems. It also activates and regulates the production of other important pregnancy hormones. Oestradiol also promotes angiogenesis and vasodilatation, which indicates a role in the regulation of human utero-placental blood flow. Indeed, this steroid induces vasodilatation of uterine and placental arteries. Oestradiol also promotes angiogenesis. In addition to these local functions, oestradiol also stimulates the proliferation of mammary epithelium, preparing the breast for breastfeeding [2,5].
Prolactin
Prolactin is the main hormone needed to trigger the production of breast milk. It enlarges the mammary glands to prepare for this. Remember, progesterone levels prevent lactation until the baby is born. Prolactin has other roles unrelated to milk production. It contributes to the development of the fetal lungs and brain, as well as the maternal immune system which tolerates the fetus [2].
Relaxin
Relaxin levels are highest during the first trimester of pregnancy, but it is present throughout. It has several roles, including prohibiting contraction of the uterine muscles to prevent premature birth. It relaxes blood vessels, increasing blood flow to the placenta and kidneys. This helps the mother’s body cope with the increased demand for oxygen and nutrients from the developing baby. Relaxin also helps the mother’s body prepare for birth. It relaxes joints in the pelvis and softens and widens the cervix to make delivery of the baby easier [2].
Oxytocin
Oxytocin only appears in significant amounts towards the end of pregnancy, although it is present in lesser amounts before that. Its levels increase when labor begins, triggering contractions. If labor does not start naturally, oxytocin can be used to induce it [2].
Hormones only Produced by Placenta
Placental lactogen
The Human Placental Lactogen (HPL), also known as human chorionic somatomammotropin, is a polypeptide hormone which assumes several growth hormone functions, including the enhancement of maternal IGF-1 synthesis.
Adiponectin
;Adiponectin is another important adipokine for placentation and gestational outcome. Adiponectin promotes insulin sensitivity, has anti-inflammatory and anti-atherogenic properties and its levels negatively correlate with body fat.
Leptin
During pregnancy, the human placenta synthesizes large amounts of leptin, which is released into maternal and fetal circulation and amniotic fluid. Leptin is mainly secreted by adipocytes into circulation, has important roles in regulation of bodyweight, appetite and energy homeostasis and its plasma levels are proportional to body fat. Moreover, this adipokine is expressed in several other tissues and organs and plays different functions, including in angiogenesis, immune function, bone metabolism and reproductive events. Leptin also seems to intervene in immunomodulation [6].
Resistin
Resistin is a cysteine-rich polypeptide that acts on insulin signaling pathways to induce insulin resistance, and acts on vascular endothelial cells and smooth muscle cells to affect cell function, suggesting that resistin may be involved in vascular lesion.
Other adipokines
In addition to, adiponectin and resistin, other adipocytederived proteins are produced by human placenta Visfatin is a newly identified adipokine, which was originally isolated from human peripheral blood lymphocytes and named pre-B-cell colony-enhancing factor [7]. It regulates lipid and carbohydrate metabolism by enhancing insulin sensitivity, decreasing triglyceride levels, in-creasing energy expenditure and causing weight loss.
Fibroblast growth factor 21 is a metabolic regulator and a plausible target for treatment of metabolic diseases. It is primarily synthesized by the liver but also expressed by adipose tissue, thymus and pancreas [8].
Pregnancy-associated plasma protein A
Pregnancy-associated plasma protein A (PAPP-A) is mainly synthesized by the syncytiotrophoblast and EVT. The role of PAPP-A during pregnancy is still poorly under-stood. Because of its protease activity, PAPP-A decreases IGFBP-4 affinity for IGF-1 and 2. Insulin-like growth factor-binding protein 4 is a protein that in humans is encoded by the IGFBP4 gene [9].
Placental protein 13
Placental protein 13 (PP13) or galectin-13 is a member of galectin superfamily. The role of PP13 during pregnancy is not fully elucidated. It is suggested that this galectin participates in immunotolerance, as PP13 seems to induce apoptosis of immune cells. The screening of maternal PP13 levels in the first trimester is a promising diagnostic tool for the prediction of preeclamp-sia with high sensitivity and specificity [10].
Kisspeptin
Kisspeptins are a family of neuropeptides. A role for kisspeptin/ Kiss1R signalling in pregnancy was already documented. In human placenta, Kp-10 inhibits in-vitro migration and invasion of trophoblasts in placental ex-plants and of primary cultures of first trimester EVT, by suppressing MMP-2 activity. Recent studies have demonstrated the involvement of the kisspeptin system in the processes of implantation and placentation [11]. In PE patients, plasma KP-10 demonstrated inverse correlation with E2 (during the 2nd trimester), LH and FSH (during the 3rd trimester) and positively correlated with β-HCG (during the 3rd trimester). Relatively high KP-10 sensitivity with the largest area under the ROC curves during 2nd and 3rd trimester of pregnancy, suggesting that is statistically acceptable as a diagnostic screening tool to rule out the PE especially in 3rd trimester [12].
Clinical Interest of Placental Hormones
Placental-related hormones play essential roles during several gestational events, including implantation, placentation, vascular remodeling, immunomodulation, labor and breastfeeding. An abnormal production of these hormones may imply significant alterations in these processes and negatively affect gestational course and fetal development. Two main anomalies have, therefore, occurred following the abnormal levels of different placental hormones and pregnancy-related conditions [13].
Pre-eclampsia
Pre-eclampsia is the major cause of maternal morbidity and mortality during pregnancy, affecting 2-8% of pregnant women. It is characterized by de-novo manifestation of hypertension (≥140/90 mmHg) and proteinuria (≥300 mg/24 h), diagnosed after the 20th week of gestation [14].
Chromosomal Anomalies
Alterations in the normal human karyotype, either in number or structure of chromosomes, may have major clinical conditions as a consequence. In fact, different types of chromosomal abnormality result in different chromosomal syndromes. Trisomy 21, also known as Down’s syndrome, is the most prevalent chromosomal anomaly and the main genetic cause of mental retardation following by, learning difficulties, a characteristic facial appearance and poor muscle tone (hypotonia) in infancy. An individual with Down syndrome has three copies of chromosome 21 rather than two. An example of monosomy, in which an individual lacks a chromosome, is Turner syndrome. In Turner syndrome, a female is born with only one sex chromosome, an X, and is usually shorter than average and unable to have children, among other difficulties [15].
Redefinition of GDM
According to the recent redefinition, GDM is characterized by insulin resistance and fasting glycaemia levels higher than 92 mg per ml [16, 17]. Regarding other adipokines, in the second trimester of GDM women, visfastin levels were higher; lower or unchanged, compared with the control group. The FGF21 levels in one study were significantly higher in women with GDM at term but other studies reported that no alterations were observed between GDM and control group [18].
Other Pregnancy-Related Conditions
Ectopic pregnancy affects about 1–2% of gestations and is generally diagnosed by transvginal pelvic sonography and physical examination [19]. This approach, however, may be questionable, and unknown location pregnancies need to be followed until its diagnosis is made. In this way, value above which an intrauterine pregnancy (IUP) should be visualized by ultrasonography evaluated, as the levels of this hormone are usually decreased in ectopic pregnancy [19]. There is ample evidence that an abnormal intrauterine environment can induce alterations in fetal metabolism with persisting consequences in late life such as [20]:
a) Austere motherly diabetes complicated by vasculopathy and nephropathy results in intrauterine growth constraint.
b) Intrauterine growth retardation is principally due to a abridged uteroplacental circulation, motherly undernutrition or malnourishment.
c) Over-stimulation of the insulin-producing B-cells may result in abridged insulin secretion in future life.
d) High glucose concentrations are known to stimulate B-cell replication, but the typical B-cell hyperplasia in fetuses of diabetic mothers only happens if the fetus has a functioning hypothalamo-hypophyseal system.
e) Fetal hyperglycaemia induces fetal hyperinsulinaemia, which is known to damage the ventromedial part of the hypothalamus, which controls insulin secretion.
f) Normalization of the diabetic intrauterine milieu in the last part of pregnancy protects B-cell function and the hypothalamus.
g) Fetal hypoinsulinaemia, resulting from hypoplasia of the endocrine pancreas in motherly and fetal undernourishment or resulting from B-cell exhaustion in austere diabetes, might have a contrasting effect.
h) Motherly diabetes connected with hyperglycemia and motherly undernourishment or abridged uteroplacental circulation associated with hypoglycemia most probably has diverse effects on fetal metabolic processes/gene expression.
.Non Hormonal roles of the Placenta and Fetus
a) Placenta supplies a growing fetus with nutrients and water.
b) Insulin resistance and the amount of insulin produced upsurges, in response to the increase of glucose concentration. In this case increased IR is linked with unsuccessful motherly and fetal outcomes [21].
c) Pancreas of the fetus senses the high glucose levels and produces more insulin in an endeavor to use this excess of glucose [21].
Factors Upsurges the Rick of GDM
Some of the factors that may increase the GDM risk include the following (22):
a) Overweight or obesity
b) Family history of diabetes
c) Having given birth previously to an infant weighing greater than 9 pounds or 4, 0824 kg
d) Age (women who are older than 25 are at a greater risk for developing gestational diabetes than younger women)
e) Race (women who are African-American, American Indian, Asian American, Hispanic or Latino, or Pacific Islander have a higher risk).
f) Prediabetes, also known as impaired glucose tolerance. Infants of mothers with gestational diabetes are vulnerable to several chemical imbalances, such as low serum calcium and low serum magnesium levels, but, in general, there are two major problems of gestational diabetes: macrosomia and hypoglycemia [22].
Macrosomia
Macrosomia talk about a baby who is significantly larger than normal. Fetus converts the superfluous glucose to fat [6]. All of the nutrients necessary for the fetus come straightforward from the mother’s blood. For pregnant diabetic women, their blood has too much glucose. If the maternal blood has too much glucose, the pancreas of the fetus senses the high glucose levels and produces more insulin in an attempt to use this glucose. Even when the mother has gestational diabetes, the fetus is able to produce all the insulin it needs. The combination of high blood glucose levels from the mother and high insulin levels in the fetus results in large deposits of fat which causes the fetus to grow excessively large.
Hypoglycemia
Hypoglycemia indicates to low blood sugar in the baby immediately after delivery. This problem occurs if the mother’s blood sugar levels have been consistently high, causing the fetus to have a high level of insulin in its circulation. After delivery, the baby continues to have a high insulin level, but it no longer has the high level of sugar from its mother, resulting in the newborn’s blood sugar level becoming very low. The baby’s blood sugar level is checked after birth, and if the level is too low, it may be necessary to give the baby glucose intravenously. Gestational diabetes is not caused by a lack of insulin, but by other hormones produced during pregnancy that can make insulin less effective, a condition referred to as insulin resistance. Do antidiabetic plants used in Cameroon useful to manage deficiency in hormones production by the placenta during GDM and by consequent insulin resistance? By having antioxidant, anti-hyperglycemic and immunosuppressive activities, these plants could be good candidates in the treatment of diabetes and diabetes in pregnancy due to hormonal dysfunction [22].
Methodology
Enclosure of plants in this study
Plants include in this study may be antidiabetic, with some of the following activities:
a) Anti-hyperglycemic,
b) Against insulin resistance
c) Insulin sensitivity
d) Antioxidant
e) Immunosuppressive
f) Regulator of placenta hormonal disorders
g) Control fetus macrosomia
h) Control pre-eclampsia condition
i) Prevent hypoglycemia in newborns
Gestational diabetes is a condition characterized by hyperglycemia that provides decreased insulin performance in its appropriate receptors by hormones secreted by the placenta and fetus. The main fetal comorbidities are macrosomia, growth disturbances, and congenital and maternal anomalies in gestational diabetes including preeclampsia, vascular lesions and postgestational diabetes.
For More Accuracy, Plants Admitted in this Study may have at Least One the Following Effects
a) Enhance a drop-in insulin performance in its appropriate receptors in gestational diabetes
b) Regulate hormones secreted by the placenta and fetus
c) Prevent macrosomia
d) Prevent growth disturbances,
Reduce Congenital and Maternal Anomalies in Gestational Diabetes Like
a) Preeclampsia
b) Hypotension
c) Vascular lesions
d) And post-gestational diabetes
Each of this second group of effects was used as engine for previous studies research on antidiabetic plants used in Cameroon. These engines are: “given plant name” enhances a drop-in insulin performance in its appropriate receptors in gestational diabetes; “given plant name” regulates hormones secreted by the placenta or fetus; “given plant name” prevents macrosomia; “given plant name” prevents growth disturbances; “given plant name” reduces congenital and maternal anomalies in gestational diabetes like (preeclampsia; hypotension; vascular lesions and post-gestational diabetes). This research was carried out in Google, Google scholar, PubMed, Only plants that are using in Cameroon will be retain our attention. The plants authentication was performed from the Project of the World Flora Online Consortium and at National herbarium of Cameroon.
Results
Advances in the control of diabetes in the decades since the discovery of insulin in the 1920’s have reduced the risk for birth imperfections by diabetic pregnant women. The update improvements of the diagnostic, the uterine and placental abnormalities, the nutritional disorders and the management of impairments on the mothers and the offsprings due to deficiency in hormones production by the placenta or fetus are presented below, following by the antidiabetic plants with potential effects for their control.
Roles of Ghrelin in the Regulation of Postnatal Growth and Several Diseases
Ghrelin has been one of the most studied intestine-derived molecules in childhood obesity. Troubles of its pathway have been projected as the basic pathophysiology for numerous diseases such as: growth hormone insufficiency, anorexia nervosis, cachexia, chronic heart failure, gastrointestinal motility conditions, osteoporosis, and obesity and Prader-Willi syndrome [23]. Ghrelin increases AMPK activity in the hypothalamus, depressing levels of malonyl∼CoA, inducing carnitine palmitoyl transferase-1, elevating long chain fatty acids, releasing NPY and stimulating hunger [24]. Ghrelin levels are inversely associated with BMI, and there is a negative connotation between insulin and this hormone. James et al. [25] informed that ghrelin levels are connected with slow weight gain from birth to 3 months of age, which links it to postnatal growth of the catch. In a study of 208 premature newborns, Darendeliler et al. [26] informed that at prepubertal age, premature newborns had higher ghrelin levels than full-term newborns [27].This sustained upsurge in ghrelin may be necessary for the compensatory growth to which they are subjected, but it does not associate with the degree of catch-up growth attained or the insulin concentrations found. Similar results were published the following year by Darendeliler et al. [28] analyzing prepubertal newborns congenital large for gestational age, recording that non-obese newborns had lower ghrelin concentrations than those born in AGA, telling that birth weight is in fact a central determinant of blood levels of ghrelin during infancy. Maffeis et al. [29] have recommended that the introduction of insulin secretion during meals promotes a reduction in ghrelin concentration and that in conditions of insulin resistance, this weakening is abridged. Nonetheless, even if this is true, this inhibitory outcome of the diet is lost in infancy, suggesting that ghrelin performances as an anabolic hormone projected to offer the substrates essential for growth [30]. This dietetic outcome on ghrelin be influenced by the accessibility of insulin and its insufficiency may explain the overeating observed in type 1 diabetes [31].
Congenital Leptin Deficiency and Polyphagia Leading to Early Obesity
Basha and Arslanian [32] conducted a trial in using overweight youngsters as subjects to evaluate the potency of insulin inhibition, recording that fasting ghrelin is determined by insulin sensitivity independent of adiposity. Obesity is a multifactorial disease that has no evident genetic cause. Obesity is known to be a hereditary disorder, with a heritability of 0.7 to 0.8 [33]. Lessons have been learned from monogenic syndromes such as congenital leptin deficiency which is characterized by overeating and early obesity. The mutations that cause this disease produce a functional protein that cannot facilitate appetite control, and patients develop binge eating very early. Persons carrying this mutation are candidates for replacing leptin because it is the defective molecule. Gibson et al. [34] informed the 4-year treatment of a subject (with the Δ133G mutation) with the subcutaneous recombinant leptin, supplying advantageous and lasting control effects on hyperinsulinemia, hyperlipidemia, mass distribution fat and TSH amounts. Not all mutations are localized to a gene and they do not have as profound an effect as the old Mendelian syndrome; most of the genes associated with obesity [35] interact with each other to express a phenotype that will end in abdominal obesity [27].
Glucose Metabolism and Onset of Obesity
Nutritional and hormonal factors can interfere with the proper development of the hypothalamus and its subsequent function, manifested by eating disorders [36,37] Women with type 1 or 2 diabetes before pregnancy have a higher risk of motherly and fetal complications than patients with gestational diabetes especially because the glucose in the 1st trimester is teratogenic and can therefore lead to malformations. “Fuel-induced teratogenesis.” is a theory that has been established to explain the teratogenic capability of glucose. Freinkel et al. have proposed that fuel in the form of hyperglycemia is the cause of diabetic embryopathy and fetopathy associated with excess fuel [38]. Various clinical discoveries have sustained this theory, particularly in some indigenous people including the Pima Indians [39,40] and Pacific Islanders [41, 42] which have the maximum prevalence of gestational diabetes effects of glucose, and macrosomia. Overnutrition is known to enhance the physiological and epigenetic resulting in chronic hyperglycemia, hyperinsulinemia and hyperleptinemia [43]. Fuel-induced cases are associated with motherly obesity and / or diabetes giving a specific metabolic profile: motherly hyperglycemia, hyperinsulinemia and low grade inflammation. Since insulin cannot cross the placental barrier, glucose is the main secretagogue in the fetal pancreas around week 27 of gestation. The development of skeletal muscles is critical for parenthood because it is responsible for the majority of the oxidation rates of glucose and fats. Macrosomal newborns have visceromegaly and large amounts of fatty tissue, but limited development of skeletal muscle, especially type II fibers which are responsible for energy production / aerobic muscle. In a fuelinducing environment, there is a divergence between myogenesis and adipogenesis, with chronic inflammation being the culprit for its interchanging undifferentiated [44-46] via reticence of AMPK, the downregulation of the pathways.
Theory Proposed by Barker: Effect of Intrauterine Growth Retardation
The Phenotypic Thrifty theory suggested by Barker [47] attempted to elucidate the relationship between intrauterine growth retardation and premature death due to cardiovascular disease or complications of T2DM. He proposed that depending on the impairment inflicted on the fetus, the programming of several axes would determine the survival of the fetus during the pregnancy phase; yet the necessary measures reproduced for salvation are harmful in parenthood. Fetal undernourishment can be realized in several ways, but the overall result is always the same: hypoglycemia and hypoxia [27].
Effects of Small Placentas
Small placentas, which have not acquired sufficient remodeling of the spiral arteries, have difficulty in oxygenating and nourishing the concept, developing hypoxic placental silence [48]. It has been proposed that changes in the adipoinsular axis of the fetus support hyperinsulinism and hyperleptinemia and afford the epigenetic work necessary to maintain this during postnatal life [49]. Small fetuses for their age are generally dysmorphic, with small bodies in proportion to their head (sparing the brain) [50], low concentrations of fat mass, and a austere response to hyperglycemia apparently due to ontogeny and a mass of small beta cells [51]. Leptin can be detected as early as 17 weeks gestation [52], modulating the development of adipose tissue. Leptin acts like the fetal life of alipostatin, giving information on maturity and fat sizes. Both in animals and humans models fetal fat exhibits features of brown and white adipose tissue, and is associated with very basic being born with a mature hypothalamic pituitary axis [53].
Growth of Adipose Tissue and the Low Amounts of Leptin It should be noted that adipose tissue grows in locules (uni and multiple) and that there is always a dominant unilocular tissue which correlates with leptin concentrations. These unilocular spots have impermanent adipocytes with abundant mitochondria, decoupling protein 1 (UCP1) and long receptor-like prolactin which is characteristics of brown tissue. They are able to secrete leptin by white adipose tissue. Lipid synthesis is a very expensive process, using 39 MJ / kg and in the fetus this path depends on the supply of oxygen and metabolic substrates [55]. In light of this, if the mother changes the amount of food and the quality of it, the proper growth of fetal adipose tissue can be modulated. Indeed, several animal models have shown that motherly chronic hypoxia, hypoglycemia and hypoinsulinemia are connected to low amounts of leptin mRNA [54-56].
Fetal Origins of Leptin and Weight of Newborns
The work of Vickers et al. [57,58] revealed that puppies born into the malnourished group were smaller at birth and had a higher food intake in the instantaneous postnatal period, and this behavior was enhanced with high calorie nourishment. They concluded that hyperinsulinism and hyperleptinemia are liable for fetal programming and overeating, obesity and high blood pressure in adults. For Ekert et al. [58] motherly nutrition during pregnancy actually reprograms leptin secretion and preserves it even in parenthood. Leptin expression was measured by determining the mRNA of the protein in the subcutaneous adipose tissue of mothers, reporting a negative correlation between birth weight and leptin rates in subcutaneous fat. Nonetheless, they were higher in pigs born to dams with late recovery, suggesting that leptin is programmed into the uterus. Thomas et al. 2001 [59] instead used the adolescent sheep model to assess the pattern of leptin secretion during pregnancy. The ewes were given a diet ranging from moderate to high. Leptin expression and protein were higher in the most overfed group, suggesting that the leptin produced was a reflection of fat deposition in the mother due to over nourishment. In addition, there was a negative correlation between motherly leptin rates and fetal and placental birthweight. In fetal sheep, leptin is known to modulate energy-consuming processes such as angiogenesis and hematopoiesis. It is primarily expressed in the brain and liver, but it can also be found in fetal skeletal muscle, kidneys, and peri-renaladipose depot. Mothers can have a negative correlation with fetal weight; nonetheless neonatal leptin rates have a positive correlation between birth weights in humans, and while altering maternal nutrition reduces serum leptin, it does not affect fetal levels [60].
Lipid Storage Capacity during Prenatal Period of Life Muhlhparausler et al. [61], confirmed these results by analyzing the effect of dietary intake on leptin levels in pregnant sheep during the second half of gestation. They revealed that cumulative motherly nourishment intake augmented glucose but not leptin levels; nonetheless, leptin was a good substitute for fetal adiposity and appear to be a moderator of endogenous energy spending in the fetus. In 2003, the same research team revealed, once again [62] that a moderate upsurge in motherly and fetal intake modulates the expression of leptin and UCP1, elucidated by the advanced capacity of unilocular fat mass to synthesize proteins; these results suggest that lipid storage capacity is established during the prenatal period of life [27].
Epigenetic Development of Pancreas and Glucose Homeostasis
The epigenetics involved in the “Small Baby” model include methylation changes in essential genes that switch beta cell ontogeny and functional differentiation [63-66]. Pancreatic and Duodenal Home box 1 (PDX1) is a transcription factor that controls the development of the growing of the bud that will become the pancreas. This factor shows a advanced decline in transcription in intrauterine growing retardation (IUGR), and it is associated with epigenetic regulation via histone methylation. GTPcyclohydrolase 1 (Gch1) is part of the folate and biopterinbiosynthesis pathways and has been positively linked to endothelial dysfunction understood in diabetes. PDX1 is liable for modulating the expression of fibroblast growth factor receptor 1 (Fgfr1) which is involved in glucose homeostasis [27].
Genetic Mechanism of Insufficient Insulin Production in Postnatal Life
In IUGR, fibroblast growth factor receptor 1 (Fgfr1) is upregulated and is connected to vessel dysfunction and pancreatic fibrosis. Lastly, IUGR is associated with an enlargement of the β cell cycle, reducing the number of mitoses, contributing to insufficient insulin production in postnatal life. The survival of the fittest fetus requires downregulation and shutdown of numerous stress and energy sensors in the liver and skeletal muscles, which is beneficial during pregnancy, but has harmful effects after pregnancy. Birth, because nutrient detection and insulin sensitivity are compromised [27,67].
Leptin Treatment of Diabetic Embryopathy and Fetopathy Related with Excess Glucose/Fuel
Leptin was considered as the support molecule according to animal and human analysis, but its place was secured once treatment with it was shown to reverse the developmental programming that occurs after motherly undernutrition during pregnancy [68]. Vickers et al. used 2.5 μg / g /d and depending on prenatal and postnatal nutritional status [69].
Dietary Treatment of Diabetic Embryopathy and Fetopathy Related with excess Glucose/Fuel
Dietary intervention trials have also been shown to be effective in reducing and / or preventing adverse effects of reprogramming, as shown by Wyrwoll et al. [70] using 3-fatty acids, and Zambrano et al. [71] (by applying a strategy of modification of the diet before pregnancy.
Intrauterine Environment and the Growth-Restricted Babies
In summary, the intrauterine environment is the key phase in acquiring the metabolic tools to survive in the motherly uterus; the problem is what happens when the baby is born? Captivatingly, these methods of survival are not without risk to the unborn fetus, as each of these conditions (IUGR and macrosomia) brings a high risk of death during birth and abrupt neonatal death [72,73]; any disparity in epigenetic composition comes at a cost, and in the world of pediatrics it is high. Growth-restricted babies are small for their gestational age, with small masses of beta cells, hypoleptinemia, and signs of “chronic hunger”, while overfed offsprings are heavy, overhead the 90th weight for their age, with a high degree of fat and reduced muscle development. Both situations revolve around leptin rates and intracellular AMPK signaling networks [27].
Increasing by Physical Exercise of β-Oxidation of Fatty Acids and Glucose Transport without Insulin Intervention
Exercise has numerous advantageous properties which makes it appropriate to include it in the day-to-day life of a highrisk child/adolescent. The primary properties of exercise include enhanced GLUT4 translocation via insulin-independent pathways which include Nitric Oxide synthesis and AMPK activation. AMPK is activated via phosphorylation of Thr172by AMPK kinase or Calmodulin dependent kinase (CaMKK). Once this enzyme is stimulated, it phosphorylates atypical Protein Kinase C(PKC) which motivates phosphatase that will act uponIRS1, leaving the insulin pathway unopposed to induce Akt and glucose transporter translocation. Moreover, AMPK induces the expression of GLUT4 gene through MEFA2A and MEFA2D (factor 2a myocyte enhancer). During AMPK activation, it will obstruct AcetilCo∼ACarboxilase 2 (ACC2), lowering Malonyl∼CoA concentrations, spiking β-oxidation of fatty acids [74].
Antidiabetic Plants for treatment of Deficiency in Hormones production by the Placenta and Fetus, Uterine and Placental Abnormalities and Glucose Teratogenic Effects
Antidiabetic plants are most frequently used in Cameroon, especially in hinterlands to manage hyperglycemia free of charge. Manifold botanicals are involved in the oral treatments in the form of decoction (Gymnema sylvestre (Retz.) R. Br. ex Schult., Spathodea campanulata P. Beauv., Catharanthus roseus Lin., Laportea ovalifolia (Schumach. & Thonn.) Chew.), maceration (Ocimum spp, Panax ginseng C.A.Mey. Allium sativum Lin. (garlic)), infusion (Opuntia spp., Plantago ovata Forssk., Basela alba Lin, Momordica charantia Lin. (Bitter melon)), powder (Mangifera indica Lin.), tisane (Camellia sinensis (L.) Kuntze); the details of ethno pharmacologiacal preparation are found in Tsabang Nole et al. 2015 [75-81]. In majority of the herbal medicines and secondary metabolites used in treating diabetes, the mechanisms of action involve regulation of insulin signaling pathways, translocation of GLUT-4 receptor and/or activation the PPARγ, etc. Several flavonoids inhibit glucose absorption by inhibiting intestinalα-amylase and α-glucosidase. In-depth studies to validate the efficacies and safeties of extracts of these traditional medicinal plants are needed, and large, well designed, clinical studies need to be carried out before the better use of such preparations can be recommended for treatment and/ or prevention of diabetes and its complications [82].
Activities of some antidiabetic plants related to gestational diabetes and its complications
Kalanchoe pinnata Lam. (Crassulaceae)
Fasting blood glucose values were reduced to 116 mg/dl from 228 mg/dl on treatment with 10 mg/kg body weight of DCM fraction, while glycated hemoglobin improved to 8.4% compared with 12.9% in diabetic controls. The insulin level and lipid profile values were close to normal values. In vitro studies demonstrated a dose-dependent insulin secretagogue action. Insulin secretion was 3.29-fold higher at 10 mg/ml as compared to the positive control. The insulin secretagogue activity was glucose independent and Kþ-ATPchannel dependent. The bioactive component of the DCM fraction was identified to be aphenyl alkyl ether derivative. The DCM fraction of Kalanchoe pinnata demonstrates excellent insulin secretagogue action and can be useful in treatment of diabetes mellitus [83].
Bryophyllum pinnatum (Crassulaceae)
The present study demonstrates the anti-diabetic property of aqueous extract of Bryophyllum pinnatum (BP) leaves using diabetic rats (albino rats) as models. At the same time, the anti-diabetic effect of the aqueous extract was compared to that of a sample containing a mixture of the extract and a commercial diabetic medicine, glibenclamide. A specified dosage of aqueous extract of Bryophyllum pinnatum (BP) leaves was administered on the experimental diabetic rats, and their BGL was measured and recorded. The results showed a significant drop in the BGL of the diabetic rats to a value close to normal blood glucose level within 120 minutes when only aqueous extract from BP leaves was used. When a sample containing a mixture of the aqueous extract and glibenclamide was administered, a further drop in BGL was observed. Therefore, the results reveal that aqueous extract of Bryophyllum pinnatum leaves have significant anti-diabetic properties, and that the performance of the existing drugs (glibenclamide) could be enhanced with the use of the aqueous extract [84].
Aloe vera (Lin.) Burm. f. (Xanthorrhoeaceae) Synonyme Aloe vulgaris Lam. (1783), Aloe barbadensis Mill. The administration of Aloe vera Lin. gel has showed a reproductive improved in PCOS rats. This result suggests that it has a protective effect. Aloe vera Lin. gel also altered the ovarian-placental steroid status by modulating the expression of Steroidogenic acute regulatory (StAR), Luteinizing hormone receptor (LHR), Androgen Receptor (AR) and Aromatase, which could also be correlated with a change in hormonal profile of important steroids. Aloe vera Lin. gel also reduces post implantation loss during gestation period leading to increased fetal viability “at term”. These modulations could be attributed to the nutritive and active ingredients present in Aloe vera gel, which independently or cumulatively act to regain fertility when used prior to conception. Thus, suggesting Aloe vera Lin gel is a good pre-conceptive agent for PCOS phenotype. Increased androgen synthesis, interrupted folliculogenesis, and insulin resistance lie at the pathophysiological fundamental of PCOS. Up-todate therapy for such a syndrome is use of insulin sensitizers. Large randomized clinical trials of metformin as the insulin-sensitizing drug, however, suggested that it produces various side effects after extended use. For this reason, an alternative therapy would be to use herbs with hypoglycemic potential. Aloe vera widely known plant is a renowned plant with such properties [85].
Allium sativum Lin. (Liliaceae) Garlic
Garlic pill prevents macromia in gestational diabetes and could reduce the effects of fasting blood sugar (FBS) and deterioration of prediabetes symptoms (primary outcomes), as well as diastolic blood pressure, neonatal anthropometric indices, and mode of delivery (secondary outcomes) in pregnant women with prediabetes [86].
Aframomum melegueta Lin. (alligator pepper)
An intra-peritoneal injection of 13.3mg/Kg body weight of aqueous extract of Aframomum melegueta showed a non-significant increase in the weight of litters from non-treated diabetic rats (p > 0.05) and a significant decrease in the weight of litters of treated diabetic pregnant rats (p < 0.05). It was concluded that Aframomum melegueta (alligator pepper) significantly reduces litter size in pregnant Sprague Dawley rats with type 1 diabetic mellitus (Ehiagwina, E. and Inegbenebor, U. 2016). Also, intraperitoneally injected with 13.3 mg/Kg body weight of aqueous extract of the seeds of alligator pepper in the first trimester of pregnancy can prevent the development of fetal macrosomia in Sprague Dawley rats [87,88].
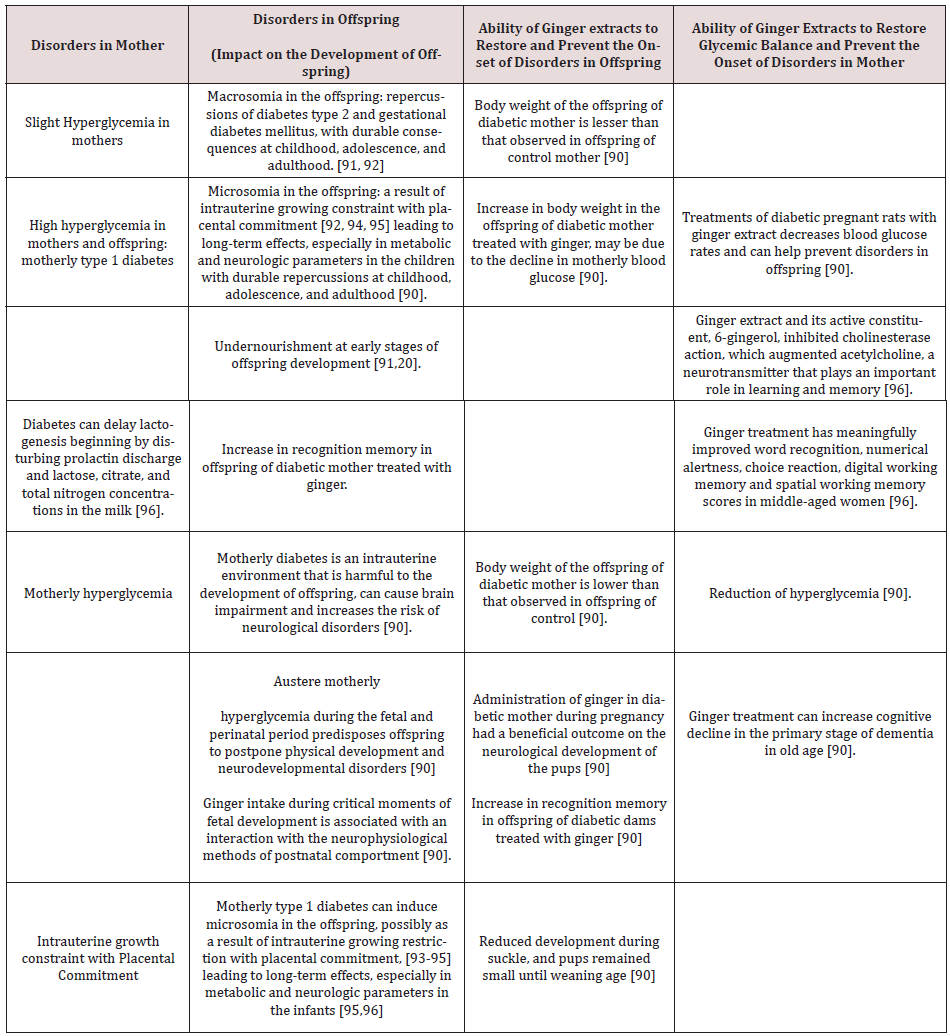
Zingiber officinale Lin. (Zingiberaceae)
Zingiber officinale extract has clearly lowed the blood glucose levels and help prevent disorders resulting to the consequences of severe maternal hyperglycemia induced by streptozotocin in Wistar rats on postnatal development of offspring.
Besides, the ability of Zingiber officinale (Ginger) extracts to restore glycemic balance in mother and prevent the appearance of disorders in offspring. Several studies showed that hyperglycemia was due to the effects of Streptozotocin by its action on pancreatic β cells, leading to a reduction of the cell mass. Indeed Streptozotocin activates the expression of protein kinase C, liable for the dephosphorylation of the insulin receptor. The treatment of diabetic pregnant rats treated as above, with Zingiber officinale (ginger) extract restores glycemic equilibrium in mother and prevents the beginning of disorders in offspring and helps to prevent the properties of severe motherly hyperglycemia induced by streptozotocin on development of offspring. Zingiber officinale components gingerols, shogaols, paradols, and zingiberene exhibit antioxidative effect, glucose and lipid lowering effects, as well as immunomodulatory, anti-inflammatory, antiapoptosis effect. The anti-inflammatory action of ginger is attributed to gingerols, shogaols, and diarylheptanoids that are thought to obstruct the activity of cyclooxygenase, inducible NO synthase and lipoxygenase suppress prostagland in synthesis and interfere in cytokine signaling [89]. The components zingerone, gingerdione and dehydrozingerones had a potent antioxidant activity. Zingiber officinale capsule was able to diminish, 2 hours after mealtimes, in women with gestational diabetes of intervention group, for 8 weeks of treatment, the mean blood glucose, the dose of insulin received, and the incidence of visits to the gynecologist, significantly compared to control group (P < 0.05) [90] What are the disorders in offspring and the effects of severe motherly hyperglycemia on the development of offspring and mother? The (Table 1) provides the answers to this question.
Table 1: Disorders related to gestational diabetes and restoration of some of them by Zingiber officinale (ginger) or its extracts
Discussion
Limitations of Modern Treatment
Diabetes is a chronic metabolic disorder that affects the quality of life in terms of physical health, social and psychological well-being. In spite of the enormous progress in the treatment of diabetes using existing commercial drugs, such as, insulin and oral hypoglycemic agents, the search for new drugs is imperative due to several limitations of the commercial drugs such as insulin resistance. In addition, the existing diabetic drugs are expensive and unaffordable by the rural population in the developing countries.
Presently, some medicinal plants are used to treat diabetes, and Zingiber officinale is one of the most potent herbs traditionally used to treat diabetes mellitus.
Different Diabetes Mellitus Treatments
In this article we endeavored to research the following types of diabetes mellitus treatments such as:
a) Hormonal,
b) Non-hormonal or oral hypoglycemiants,
c) Medicinal plants,
d) Nanotechnology,
e) And phyto-nanotechnology.
Herbal medicines are typically used to treat diabetes mellitus by indigenous people in rural areas especially in Cameroon; because of the accessibility of a huge number of medicinal plants in the vast and diverse ecosystems tropical.
The Seven Types of Non-Hormonal Antihyperglycemic Medications Currently Accepted for the Treatment of Diabetes Are
a) Biguanides,
b) Sulfonylureas,
c) Meglitinides,
d) Glitazones,
e) Alpha-glucosidase (AGI) inhibitors,
f) Dipeptidyl peptidase-4 inhibitors ( DPP-4),
g) and sodium-glucose co-transporter inhibitors.
Uses of these categories of antidiabetic drugs bring risks, limitations, and side effects when recommended in certain conditions in comparison to management with hormones like insulin and incretin-based hormonal therapy. In the present study, an effort has been made to explore the antidiabetic medicinal plants, which may be beneficial to the scientists, health professionals, and research scholar, operational in the field of pharmacology and antidiabetes mellitus drugs discovery. Zingiber officinale, Kalanchoe pinnata Lam. et Aframomum melegueta Lin. can be exploited by pharmacologists.
Contra-indications
Antidiabetic plants containing constituents with reverse effects in pregnancy like prostaglandins must be avoided. Undeniably prostaglandins cause powerful contractions of the uterus that lead eventually to ejection of the fetal or embryonic tissue, but their effects on smooth muscle elsewhere in the body lead to such worrying side effects like vomiting and diarrhea. The dose 20 gm of prostaglandins invaginally 3h after aborts 80% of pregnancies or augmented cesarian section rates. Antimetabolites such as methotrexate inhibit DNA synthesis in rapidly dividing cells such as the trophoblast, resulting in release of prostaglandins and cytokines, extravasation of blood into the decidua, uterine contractions, and ejection of embryonic or fetal tissue. Drugs that obstruct the synthesis of progesterone (epostane) or block its receptors (mifepristone) reverse the dominant influence of progesterone during pregnancy. The dose of 200 mg per 7 days at 5-8 weeks causes 84% to terminate within 2 weeks, usually at 5 days of 2nd trimester pregnancy. As a result, a cascade of events is initiated, including influx of leukocytes and red blood cells into the decidua, release of prostaglandins and cytokines, and uterine contractions. Addition of uterotonic agents such as prostaglandins results in powerful uterine contractions, which supplement those induced by the withdrawal of progesterone. Because these methods reproduce many of the same physiological changes, clinical management of medical abortion is similar to that of spontaneous abortion. Some prostaglandin-like compounds have also been isolated in plants such as Chromolaena spp (Asteraceae) Carex aquatilis (Cyperaceae) and Linum usitatissimum (Linaceae) water sedge; and direct precursors (arachidonic acid, di-homo-γ- linolenic acid and eicosapentaenoic acid) have been discovered in a variability of plants and microorganisms, including certain red, brown algae [91-92]. These methods provide a useful alternative to surgical abortion in early pregnancy [93].
The use of nanomedicine in diabetic patients is in the initial stages, but it is may be rapidly progressing. Nanomaterials have exceptional physicochemical effects, such as high surface to mass ratio, ultra-small size, and high reactivity; these properties can be used to overcome the restrictions of traditional DM managements and diagnostic [96].
Conclusion
In term of this work, we notice that pregnancy is a physiological state characterized by drastic changes in the hormonal profile. In fact, during this period, placenta and fetus synthesize and secretes several hormones that are crucial for the regulation of distinct pregnancy stages, such as decidualization and implantation and labor, and also for the maternal metabolic adaptation and preparation for breastfeeding. Nevertheless, each of these hormones is tightly regulated in space and time by several factors, including by other placental hormones. On the other hand, altered levels or deficiency in production of placenta and fetal hormones negatively affect different pregnancy processes, and produce poor gestational outcome including microsonia and macrosomia. The deficient production of these hormones may also contribute to the altered placental growth observed in these conditions. Indeed, abnormal levels of hormones cause several pregnancy-related diseases like gestational diabetes. However, reliable biomarkers are needed for diseases linked to endocrine alterations and other pathologies.
In addition to hormonal deficiency, malformations related to the intrauterine environment such as the small placenta and the growth retardation of the uterus, glucose teratogenesis, the growth of adipose tissues, the ability to store lipids during the prenatal period, Chromosomal anomalies and nutritional disorders can be detrimental to the development of pregnancies and prenatal life. The use of certain hormones likes leptin, etc. and physical exercises can provide important corrections. The present study shows that an effort has been made to explore the antidiabetic medicinal plants, which may be beneficial to the scientists, health professionals, and research scholar, operational in the field of pharmacology and antidiabetes mellitus drugs discovery. Zingiber officinale, Kalanchoe pinnata Lam. et Aframomum melegueta Lin. can be exploited for by pharmacologists.
Acknowledgment
The emotional and encouraging assistance from my family is greatly acknowledged.
References
- Eman M Alfadhli (2015) Gestational diabetes mellitus. Saudi Med J 36(4): 399-406.
- https://www.compoundchem.com/2019/02/28/pregnancy-hormones/
- Gresso S, C Palumbo Rusol0 S, Cianci LA, Tumino G, Reitano G (1980) Human fetal insulin secretion in response to maternal glucose and leucine administration. Pediat Rear 14(5): 782-783.
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/diabetes/gestational-diabetes
- Mario Ciampelli, Antonio Lanzone, Alessandro Caruso (1998) Insulin in obstetrics: A main parameter in the management of pregnancy. European Society of Human Reproduction and Embryology 4(6): 904-914.
- Hyeong Kyu Parka, Rexford S Ahima (2015) Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism Clinical and Experimental 64(1): 24-34.
- Song H, QX Lin, RA Li, K Xiao, KM Peng (2014) The expression of visfatin in mouse ovary and its regulatory effect on IFN-γ. Pak Vet J 34(2): 180-184.
- Tetsuya Nishimura, Yuhki Nakatake, Masakazu Konishi, Nobuyuki Itoh (2000) Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta 1492(1): 203-206.
- Leguy, Stephanie Brun, Guillaume Pidoux, Houria Salhi, Agnes Choiset, et al. (2014) Pattern of secretion of pregnancy-associated plasma protein-A (PAPP-A) during pregnancies complicated by fetal aneuploidy, in vivoandin vitro. Reproductive Biology and Endocrinology 129.
- Kai Lun Hu, Hsun Ming Chang, Hong Cui Zhao, Yang Yu, Rong Li, et al. (2019) Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Human Reproduction Update, Vol.25, No.3 pp. 326–343, 2019
- Hisham Al Matubsi, Farqad B Hamdan, Maysoun A Al Kaabi (2018) Evaluation of maternal plasma kisspeptin-10 and its relation to altered reproductive hormones in preeclamptic pregnant women. J Women's Health Care, Fetal and Maternal Medicine 7: 48.
- Serif Kavvasoglu, Zehra Sema Ozkan, Banu Kumbak, Mehmet Sımsek, Necip Ilhan (2012) Association of kisspeptin-10 levels with abortus imminens: A preliminary study Alternative Fetal-Medicine. Arch Gynecol Obstet (2012) 285: 649-653.
- Napso T, Yong HEJ, Lopez Tello J, Sferruzzi Perri AN (2018) The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Frontiers Physiology 9: 1091.
- Jennifer Uzan, Marie Carbonnel, Jean Marc Ayoubi (2011) Pre-eclampsia: Pathophysiology, diagnosis, and management. Vascular Health and Risk Management 7: 467-474.
- https://www.genome.gov/about-genomics/fact-sheets/Chromosome-Abnormalities-Fact-Sheet
- Joshua H Barash, Edward M Buchanan, Christina Hillson (2014) Diagnosis and Management of Ectopic Pregnancy. Am Fam Physician 90(1): 34-40.
- Bee K Tan, Kavitha Sivakumar, Harpal S Randeva (2013) Lower Cerebrospinal Fluid/Plasma Fibroblast Growth Factor 21 (FGF21) Ratios and Placental FGF21 Production in Gestational Diabetes. PLoS One 8(6): 65254.
- Holemans K, L Aerts, FA Van Assche (2003) Symposium Report Lifetime consequences of abnormal fetal pancreatic development. J Physiol 547(1): 11-20.
- Sonagra Amit D, Shivaleela M, Biradar Dattatreya K, Jayaprakash Murthy DS (2014) Normal Pregnancy- A State of Insulin Resistance. J Clin Diagn Res 8(11): 1-3.
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/diabetes/gestational-diabetes
- F Cordido, ML Isidro, R Nemina, S Sangiao Alvarellos (2009) Ghrelin and growth hormone secretagogues, physiological and pharmacological aspect. Current Drug Discovery Technologies 6(1): 34-42.
- M Gil Campos, CM Aguilera, R Canete, A Gil (2006) Ghrelin: A hormone regulating food intake and energy homeostasis. British Journal of Nutrition 96(2): 201-226.
- RJA James, RF Drewett, TD Cheet ham (2004) Low cord ghrelin levels in term infants are associated with slow weight gain over the first 3 months of life. Journal of Clinical Endocrinology and Metabolism 89(8): 3847-3850.
- HHM Maes, MC Neale, LJ Eaves (1997) Genetic and environmental factors in relative body weight and human adiposity. Behavior Genetics 27(4): 325-351.
- Joselyn Rojas, Nailet Arraiz, Miguel Aguirre, Manuel Velasco, Valmore Bermudez (2011) AMPK as Target for Intervention in Childhood and Adolescent Obesity. Hindawi Publishing Corporation. Journal of Obesity.
- WT Gibson, IS Farooqi, M Moreau (2004) Congenital leptin deficiency due to homozygosity for the Δ133G mutation: report of another case and evaluation of response to four years of leptin therapy. Journal of Clinical Endocrinology and Metabolism 89(10): 4821-4826.
- F Darendeliler, F Bas, R Bundak (2008) Elevated ghrelin levels in preterm born children during prepubertal ages and relationship with catch-up growth. European Journal of Endocrinology. 159(5): 555-560.
- F Darendeliler, S Poyrazoglu, F Bas, O Sancakli, G Gokcay, (2009) Ghrelin levels are decreased in non-obese prepubertal children born large for gestational age. European Journal of Endocrinology (6): 951-956.
- C Maffeis, RC Bonadonna, A Consolaro (2006) Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. European Journal of Endocrinology 154(1): 61-68.
- S Bellone, N Castellino, F Broglio (2004) Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. Journal of Clinical Endocrinology and Metabolism 89(4): 1662-1665.
- G Murdolo, P Lucidi, C Di Loret (2003) Insulin is required for prandial ghrelin suppression in humans. Diabetes 52(12): 2923-2927.
- WT Gibson, IS Farooqi, M Moreau (2004) Congenital leptin deficiency due to homozygosity for the Δ133G mutation: Report of another case and evaluation of response to four years of leptin therapy. Journal of Clinical Endocrinology and Metabolism 89(10): 4821-4826.
- SG Bouret (2010) Role of early hormonal and nutritional experiences in shaping feeding behavior and hypothalamic development. Journal of Nutrition 140(3): 653-657.
- Coupe B, V Amarger, I Grit, A Benani, P Parnet, et al. (2010) Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 151(2): 702-713.
- N Freinkel (1980) Of pregnancy and progeny. Diabetes 29(12): 1023-1035.
- J Pettitt, WC Knowler, HR Baird, PH Bennett (1980) Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care 3(3): 458-464.
- PW Franks, HC Looker, S Kobes ( 2006) Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 55(2): 460-465.
- L Touger, HC Looker, J Krakoff, RS Lindsay, V Cook, et al. (2005) Early growth in off spring of diabetic mothers. Diabetes Care 28(3): 585-589.
- DR McCance, DJ Pettitt, RL Hanson, LTH Jacobsson, WC Knowler, PH Bennett (1994) Birth weight and noninsulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype?. British Medical Journal 308(6934) 942-945.
- JA Westgate, RS Lindsay, J Beattie (2006) Hyperinsulinemia in cord blood in mothers with type 2 diabetes and gestational diabetes mellitus in New Zealand. Diabetes Care 29(6): 1345-1350.
- AK Rao, K Daniels, YY El-Sayed, MK Moshesh, AB Caughey (2006) Perinatal outcomes among Asian American and Pacific Islander women. American Journal of Obstetrics and Gynecology (3): 834-838.
- D Simmons, BH Breier (2002) Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care 25(9): 1539-1544.
- DJP Barker (1995) Fetal origins of coronary heart disease. British Medical Journal 311(6998): 171-174.
- P Fleming, WY Kwong, R Porter (2004) The embryo and its future. Biology of Reproduction 71(4): 1046-1054.
- MH Vickers, S Reddy, BA Ikenasio, BH Breier (2001) Dysregulation of the adipoinsular axis a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. Journal of Endocrinology 170(2): 323-332.
- SJ Roza, EAP Steegers, BO Verburg (2008) What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. American Journal of Epidemiology 168(10): 1145-1152.
- J Rozance, SW Limesand, JS Barry, LD Brown, WW Hay Jr (2009) Glucose replacement to euglycemia causes hypoxia, acidosis and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatric Research 65(1): 72-78.
- D Jaquet, J Leger, C Levy-Marchal, JF Oury, P Czernichow (1998) Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. Journal of Clinical Endocrinology and Metabolism 83(4): 1243-1246.
- IC McMillen, BS Muehlhauser, JA Duffield, BSJ Yuen (2004) Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proceedings of the Nutrition Society 63(3): 405-412.
- MJ Zhu, B Han J Tong (2008) AMP-activated protein kinase signaling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over nourished sheep. Journal of Physiology 586(10): 2651-2664.
- JM Wallace, RP Aitken, JS Milne, WW Hay Jr (2004) Nutritionally mediated placental growth restriction in the rowing adolescent: Consequences for the fetus. Biology of Reproduction 71(4): 1055-1062.
- D Jaquet, J Leger, C Levy Marchal, JF Oury, P Czernichow (1998) Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. Journal of Clinical Endocrinology and Metabolism 83(4): 1243-1246.
- MH Vickers, BH Breier, WS Cutfield, PL Hofman, PD Gluckman (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. American Journal of Physiology 279(1): 83-87.
- E Ekert, KL Gatford, BG Luxford, RG Campbell, PC Owens (2000) Leptin expression in offspring is programmed by nutrition in pregnancy. Journal of Endocrinology 165(3): 1-6.
- L Thomas, JM Wallace, RP Aitken, JG Mercer, P Trayhurn, et al. (2001) Circulating leptin during ovine pregnancy in relation to maternal nutrition, body composition and pregnancy outcome. Journal of Endocrinology 169(3): 465-476.
- RA Ehrhardt, A Bell, YR Boisclair (2002) Spatia land developmental regulation of leptinin fetal sheep. American Journal of Physiology 282(6): 1628-1635.
- BS Muhlhausler, CT Roberts, JR Mc Farlane, KG Kauter, IC McMillen (2002) Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biology of Reproduction 67(2): 493-499.
- A Strobel, T Issad, L Camoin, M Ozata, AD Strosberg (1998) A leptin missense mutation associated with hypogonadism and morbid obesity. Nature Genetics 18(3): 213-215.
- LP Erusse, T Rankinen, A Zuberi (2005) The human obesity gene map: The 2004 update. Obesity Research 13(3): 381-490.
- HV Petersen, M Peshavaria AA, Pedersen (1998)Glucose stimulates the activation domain potential of the PDX-1 homeodomain transcription factor. FEBS Letters 431(3): 362-366.
- SW Limes, J Jensen JC, Hutton, WW Hay (2005) Diminished β-cell replication contributes to reduced β- cell mass in fetal sheep with intrauterine growth restriction. American Journal of Physiology 288(5): 1297-1305.
- SR Thorn, TRH Regnault, LD Brown (2009) Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150(7): 3021-3030.
- MH Vickers, PD Gluckman, AH Coveny (2005) Neonatal leptin treatment reverses developmental programming. Endocrinology 146(10): 4211-4216.
- MH Vickers, PD Gluckman, AH, Coveny (2008) The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology 149(4): 1906-1913.
- CS Wyrwoll, PJ Mark, TA Mori, IB Puddey, BJ Waddell (2006) Prevention of programmed hyperleptinemia and hypertension by postnatal dietary ω-3 fatty acids. Endocrinology 147(1): 599-606.
- E Zambrano, PM Martinez Samayoa, GL Rodriguez Gonz alez, PW Nathanielsz (2010) Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. Journal of Physiology 588(10): 1791-1799.
- R Resnik (2002) Intrauterine growth restriction. Obstetrics and Gynecology 99(3): 490-496.
- F Galtier Dereure, C Boegner, J Bringer (2000) Obesity and pregnancy: Complications and cost. American Journal of Clinical Nutrition 71(5): 1242-1248.
- CT Lim, B Kola, M Korbonits (2010) AMPK as a mediator of hormonal signaling. Journal of Molecular Endocrinology 44(2): 87-97.
- Nol Tsabang, Stella Kadjob, Rose N Mballa, Clement G Yedjou, Nga Nnanga, N, et al. (2015). New approach for the development of improved traditional medicine: case of a preparation of an oral hypoglycemic medicine from Laportea ovalifolia (Schumach. & Thonn.) Chew. (Urticaceae) Molecular Pharmaceutic & Organic Prossess Research 3(2): 125.
- Nole T, Lionel TDW, Cedrix TFS, Gabriel AA (2016) Ethnomedical and Ethnopharmacological Study of Plants Used for Potential Treatments of Diabetes and Arterial Hypertension by Indigenous People in Three Phytogeographic Regions of Cameroon. Diabetes Case Rep 1:110.
- Tsabang N, Tsambang DW L (2017) A Different Approach in the Traditional Treatment of Diabetes and of Antidiabetic Plants Discovery in Cameroon. Glob J Pharmaceu Sci 3(4): 1-5.
- Armelle D Tchamgoue, Lauve RY Tchokouaha, Tsabang Nolé, Protus Arrey Tarkang, Jules-Roger Kuiate, et al. (2018) Costus afer protects cardio, hepato and reno antioxidant status in streptozotocin-intoxicated Wistar rats. BioMed Research International.
- Tsabang N, Djeufack LWT, Yedjou CG, Tchounwou PB (2019) Importance of food plants in the prevention and treatment of diabetes in Cameroon. Bioactive Compounds in Health and Disease 2(2): 11-26.
- Nole T, Wilfried Lionel TD (2020) Conditions for Better uses of Some Cameroonian Plants Potentially Anti-Diabetic and Reversing Insulin Resistance. Diabetes Obes Int J 5(1): 1-15.
- Nolé T, Lionel TDW (2021) Contribution to the Management of Abnormal Insulin Secretion in Diabetes of Pregnancy using Antidiabetic Medicinal Plants in Cameroon. Diabetes and Obesity international journal 6(1): 1-8.
- Ota A, Ulrih NP (2017) An Overview of Herbal Products and Secondary Metabolites Usedfor Management of Type Two Diabetes. Frontiers Pharmacology 8: 436.
- Swapnil B Patil, Vandana R Dongare, Chaitanya R Kulkarni, Madhav M Joglekar, Akalpita U Arvindekar (2013) Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharm Biolology 51(11): 1411-1418.
- Gurnani C, Kumar V, Mukhija S, Narula P,Bhola R (2014) Conservation of medicinal plant Bryophyllum pinnatum (Lam.) Kurz by in vitro nodal culture. Int J Pharm Sci Res 5(6): 2406-2411.
- Radha Maharjan, Padamnabhi S Nagar, Laxmipriya Nampoothiri (2010) Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. Journal of Ayurveda & Integrative Medicine 1(4): 273-279.
- Farnaz Faroughi, Sakineh Mohammad-Alizadeh Charandabi Yousef Javadzadeh, Mojgan Mirghafourvand (2017) Effects of Garlic Pill on Blood Glucose Level in Borderline Gestational Diabetes Mellitus: A Randomized Controlled Trial. Ran Red Crescent Med J In Press(InPress): 60675.
- Ehiagwina E, Inegbenebor U (2016) Effect of apueous extract of alligator pepper (Aframomum melegueta (Zingiberaceae)) on litter size of alloxan induced diabetic Sprague Dawley rats. International journal of community research 5(1): 62-66
- Ute Inegbenebor, Festus Eghomwanre (2017) Effect of Alligator Pepper on Litter Weight of Rats Fed on High Glycemic Index Diet. Food and Nutrition Sciences 8(8): 793-800.
- Zohre Bahramian ID, Fahimeh Sehhatie-Shafaie ID, Mojgan Mirghafourvand, Shamsi Abbasalizadeh, Yusuf Javadzadeh (2017) The Effect of Ginger Capsules on the Control of Blood Sugar in Gestational Diabetes: A Triple-Blind Randomized Controlled Clinical Trial. Crescent Journal of Medical and Biological Sciences 5(4): 358-365.
- Mehouel Raouia, Ferhati Habiba, Tahraoui Abdelkrim (2020) Effect of Ginger on Hyperglycemia Induced by Streptozotocin in Pregnant Rats and Postnatal Neurodevelopment of their Offspring. Acta Pharm Sci 58(1): 101.
- Ornoy A (2011) Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Re-prod Toxicol 32(2): 205-212.
- Rudge MV, Piculo F, Marini G, Damasceno DC, Calderon IM, et al. (2013) Translational research in gestational diabetes mellitus and mild gestational hyperglycemia: current knowledge and our experience. Arq Bras Endocrinol Metabol 57(7): 497-508.
- Van Assche FA, Holemans K, Aerts L (2001) Long-term consequences for offspring of diabetes during pregnancy. Br Med Bull 60: 173-182.
- Rudge MV, Piculo F, Marini G, Damasceno DC, Calderon IM, et al. (2013) Translational research in gestational diabetes mellitus and mild gestational hyperglycemia: Current knowledge and our experience. Arq Bras Endocrinol Metabol 5(7): 497-508.
- Volpato GT, Damasceno DC, Sinzato YK, Ribeiro VM, Rudge MV, et al. (2015) Oxidative stress status and placental implications in diabetic rats undergoing swimming exercise after embryonic implantation. Reprod Sci 22(5): 602-608.
- Hartmann P, Cregan M (2001) Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J Nutr 131(11): 3016-3020.
- Saenghong N, Wattanathorn J, Muchimapura S, Tongun T, Piyavhatkul N, et al. (2012) Zingiber officinale improves cognitive function of the middle-aged healthy women. Evid Based Complement. Alternat. Med 1-9.
- Saenghong N, Wattanathorn J, Muchimapura S, Tongun T, Piyavhatkul N, et al. (2012) Zingiber officinale improves cognitive function of the middle-aged healthy women. Evid Based Complement Alternat Med 1-9.
- Perna R, Loughan AR, Le J, Tyson K (2015) Gestational diabetes: long-term central nervous system developmental and cognitive sequelae. Applied Neuropsychol Child 4(3): 217-220.
- Hami J, Shojae F, Vafaee Nezhad S, Lotfi N, Kheradmand H, et al. (2015) Some of the experimental and clinical aspects of the effects of the maternal diabetes on developing hippocampus. World J Diabetes 6(3): 412-422.
- Amarvani P Kanjikar (2019) On Anti-Diabetic Potential of Phyto-nanoparticles Comparison with Hormonal Therapy and Medicinal Plants. International Journal of Pharmaceutical and Phytopharmacological Research (eIJPPR) 9(1): 103-111.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




