
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Review Article(ISSN: 2638-5910) 
Pathophysiology of Cardiovascular Complications in Obesity and Diabetes Volume 3 - Issue 3
Naranjan S Dhalla1*, Paramjit S Tappia2 and Anureet K Shah3
- 1Institute of Cardiovascular Sciences, St. Boniface Hospital Albrechtsen Research Centre & Department of Physiology and Pathophysiology, College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada
- 2Asper Clinical Research Institute, St. Boniface Hospital, Winnipeg, Canada
- 3Department of Kinesiology, Nutrition and Food Science, California State University, Los Angeles, USA
Received:April 22, 2021; Published: April 29, 2021
Corresponding author: Naranjan S Dhalla, St. Boniface Hospital Albrechtsen Research Centre, Canada
DOI: 10.32474/ADO.2021.03.000163
Introduction
According to the World Health Organization (WHO), the global incidence of obesity has nearly tripled since 1975; more than 1.9 billion adults were overweight and 650 million of those were obese in 2016. The WHO has defined obesity as an abnormal or excessive accumulation of fat that may impair health [1]. On the other hand, the number of people with diabetes rose from 108 million in 1980 to 422 million in 2014. Between 2000 and 2016, there was a 5% increase in premature mortality from diabetes and in 2019, an estimated 1.5 million deaths were directly caused by diabetes. Another 2.2 million deaths were attributable to high blood glucose in 2012 [2]. It is without any ambiguity that both these chronic metabolic diseases represent universal major health hazards and are on the rise in both developed and developing nations. It should be pointed out that type 1 diabetes involves the lack of production of insulin, whereas type 2 diabetes is due to an insulin resistance. Both types of diabetes as well as obesity lead to several health complications, including cardiovascular disease. Several books are available that provide a wealth of experimental and clinical lines of evidence indicating that both diabetes and obesity are major risk factors for the development of heart disease [3-8]. In this article we describe some of the common characteristics and features in the etiology of cardiovascular abnormalities associated with obesity and diabetes-induced cardiomyopathies [9,10].
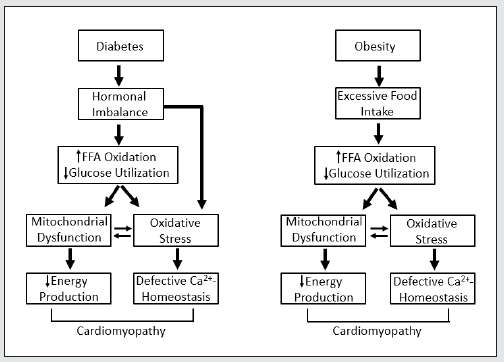
Obesity cardiomyopathy occurs in individuals with severe and long-standing obesity that can lead to progressive congestive heart failure and increased risk of sudden cardiac death [9]. In this regard, there is an increase in total blood volume and cardiac output because of the high metabolic activity of excessive fat in obesity [9]. In moderate to severe cases of obesity this can lead to an increase in left ventricle (LV) dilation, increase in LV wall stress, compensatory LV hypertrophy and LV diastolic dysfunction. If the LV wall stress remains high then LV systolic dysfunction occurs. Likewise, diabetic cardiomyopathy presents with reduced LV ejection fraction, augmented pre-ejection time and increased LV end-diastolic pressure due high LV wall stiffness and isovolumic relaxation as well as depressed cardiac contractility [10,11]. The similarities in the major biochemical mechanisms in both diabetes and obesity are shown in (Table 1). It is our contention that the main upstream factors that lead to heart dysfunction are hormonal imbalance in diabetes-induced heart disease and excessive food intake in obesity-induced heart disease. Figure 1 shows the key downstream sequence of events that ultimately lead to cardiomyopathy in diabetes and obesity in these two metabolic diseases. It is noteworthy that sympathetic outflow is increased in both diabetic and obese subjects [11,12]. Oxidative stress and inflammation are considered to play critical roles in inducing cardiomyopathy as well as insulin resistance in diabetes and obesity [11,13-15]. Oxidative stress, inflammation and mitochondrial dysfunction (including reactive oxygen species production and energy deficiency) may be due to the effects of the upregulation of the renin angiotensin system (RAS) in these metabolic diseases [16,17].
Hyperglycemia has also been identified as the key determinant in the development of oxidative stress and cardiomyopathy in both diabetes and obesity [18,19]. The development of oxidative stress may induce defects in cardiomyocyte Ca2+-handling that attenuate cardiac function because dysregulation of Ca2+-homeostasis is known to occur in both diabetic cardiomyopathy and obesity cardiomyopathy [11,20,21]. It is pointed out that although the molecular and cellular aspects of diabetic cardiomyopathy have been studied extensively, there is relatively a paucity of information regarding the mechanisms involved in the pathogenesis of obesity cardiomyopathy and thus needs to be further explored. Furthermore, nutritional approaches involving macronutrients, have been well documented for the management and treatment of both obesity and diabetes. However, the potential of micronutrients, particularly as many of them exhibit antioxidants/anti-inflammatory actions [11,13,22], as well as pharmacological agents that exert dual antioxidant and anti-inflammatory actions [23] should be explored in preclinical trials in these pathological conditions.
Table 1: Pathogenic mechanisms that lead to cardiomyopathy in chronic obesity and diabetes.
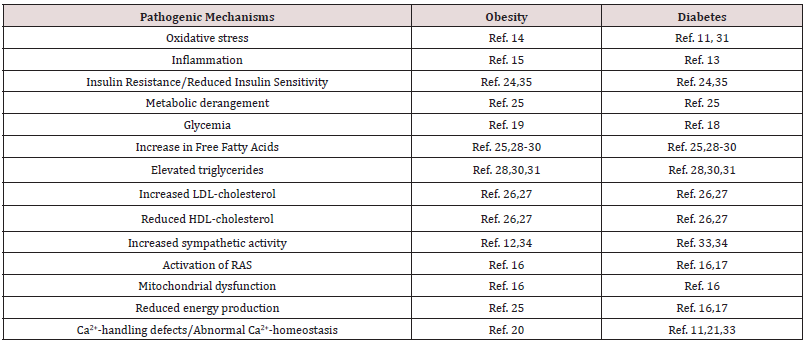
Ref. number for each mechanism in obesity or diabetes refer to those cited in the Reference Section.
Figure 1: Schematic representation of events involved in the pathogenesis of cardiomyopathy in chronic diabetes and obesity. Hormonal imbalance in diabetes refers to the changes in plasma levels of insulin deficiency or resistance in addition to the elevated levels of other hormones such as catecholamines and angiotensin II, which are known to promote oxidative stress. FFA, free fatty acids; - increase; - decrease.
Body mass index is related to diabetes and insulin resistance, in fact, both type 2 diabetes and obesity are associated with insulin resistance [24]. Both diabetes and obesity induce a distinct cardiac metabolic phenotype that underlie the functional abnormalities of the heart [25]; dyslipidemias are alterations to the plasma lipid profile that are often associated with both metabolic diseases [26,27]. Most patients with obesity and diabetes have hypertriglyceridemia and increased plasma levels of fatty acids (FA), which are taken up and stored in lipid droplets in the heart. Intramyocardial lipids that exceed the capacity for storage and oxidation can be lipotoxic and induce non-ischemic and nonhypertensive cardiomyopathy [28]. Type 2 diabetes and obesity are associated with systemic inflammation, generalized enlargement of fat depots and uncontrolled release of FA into the circulation [29]. The heart balances uptake, metabolism and oxidation of FA to maintain ATP production, membrane biosynthesis and lipid signaling. Under conditions where FA uptake outpaces FA oxidation and FA sequestration as triacylglycerols in lipid droplets, toxic FA metabolites such as ceramides, diacylglycerols, long-chain acyl- CoAs, and acylcarnitines can accumulate in cardiomyocytes and cause cardiomyopathy [30,31].
Metabolic derangement results in an increase in FA uptake and β-oxidation in the heart. These changes in FA metabolism have a negative effect on cardiac contractile function in both diabetes and obesity; the relation and the mechanisms of myocardial FA metabolism have been highlighted in an excellent review article by Lopaschuk et al, [25]. In addition, these researchers have examined the potential of targeting of FA metabolism as a therapeutic intervention for heart disease, which could be an important approach for the treatment/prevention of heart disease in high risk diabetic and obese individuals. It is interesting to note that following the onset of heart failure, obesity per se is associated with preserved tissue ATP and mitochondrial oxyradical production, which has been suggested to be a favorable metabolic pattern for survival [32]. It is also pointed out that sympathetic overdrive contributes to the derangement of glucose metabolism in clinical conditions such as obesity and type 2 diabetes and the increased cardiac levels of catecholamines and production of catecholamine oxidation products have been shown to result in metabolic derangements and Ca2+ -handling abnormalities [33]. Thus, targeting of the sympathetic nervous system directly may prove to be highly beneficial; an approach that could be incorporated in routine clinical practice [34].
Interestingly, there occurs a close relationship between obesity and diabetes because the metabolic derangement in obesity can be seen to lead to the occurrence of diabetes, whereas similar metabolic derangement in diabetes may result in obesity. An excellent editorial by Robert Lustig [35] has addressed this very important question of the sequence of events for insulin resistance with respect to the time-course for diabetes and obesity. It has also been argued that resistance to insulin action starts in the liver and then the pancreas secretes more insulin to make the liver do its job; this raises insulin levels in the body, promotes adipogenesis, and increases peripheral insulin resistance all at the same time. However, when the hypothalamus becomes insulin resistant first, the leptin signal is antagonized resulting in increased appetite and weight gain and eventually peripheral insulin resistance. The role of the hypothalamus-leptin axis with respect to insulin resistance and the relationship with diabetes and obesity is an important area of research and needs to be further investigated [36].
Conclusion
In conclusion, we have attempted to show that there are several common concepts and mechanisms that result in cardiomyopathy in individuals with obesity and/or diabetes. While in chronic conditions of both these metabolic diseases, the occurrence of oxidative stress and inflammation are major factors that cause insult on cardiac function. Thus, targeting these factors may prove to be highly beneficial in the prevention of downstream defects that lead to functional abnormalities in the heart in diabetes and obesity. It should also be mentioned that sex differences and ethnic variations are known to exist in the incidence of both diabetes and obesity and thus any approach to understand the etiology and disease outcomes should take these important aspects into consideration. Nonetheless, the present discussion regarding the overlap in the pathogenic mechanisms of cardiomyopathy in obesity and diabetes is indicative of their co-existence and provide scope for the examination on the cause-effect relationship between these metabolic diseases.
Acknowledgement
Infrastructural support for this work was provided by the St. Boniface Hospital Albrechtsen Research Centre.
References
- https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- https://www.who.int/news-room/fact-sheets/detail/diabetes
- Tappia PS, Ramjiawan B, Dhalla NS (2020) Pathophysiology of Obesity-Induced Health Complications. Springer Nature 19: 1-345.
- Tappia PS, Bhullar SK, Dhalla NS (2020) Biochemistry of Cardiovascular Dysfunction in Obesity. Springer Nature 1-401.
- Tappia PS, Ramjiawan B, Dhalla NS (2021) Cellular and Biochemical Mechanisms of Obesity. Springer Nature, Switzerland.
- Pierce GN, Beamish RE, Dhalla NS (1988) Heart Dysfunction and diabetes. CRC Press, USA.
- Nagano M, Dhalla NS (1991) The Diabetic Heart. Raven Press Ltd. London, UK.
- Turan B, Dhalla NS (2014) Diabetic Cardiomyopathy. Springer Nature, Switzerland.
- Alpert MA (2001) Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am J Med Sci 3214): 225-236.
- Regan TJ, Ahmed S, Haider B, Moschos C, Weisse A (1994) Diabetic cardiomyopathy: Experimental and clinical observations. N Engl J Med 91(11):776-778.
- Dhalla NS, Shah AK, Tappia PS (2020) Role of oxidative stress in metabolic and subcellular abnormalities in diabetic Int J Mol Sci 21(7): 2413.
- Eikelis N, Esler M (2005) The neurobiology of human obesity. Exp Physiol 90(5): 673-682.
- Bartekova M, Radosinska J, Jelemensky M, Dhalla NS (2018) Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev 23(5): 733-758.
- Gutierrez Cuevas J, Sandoval Rodriguez A, Meza Rios A, Monroy Ramirez HC, Galicia Moreno M, et al (2021) Molecular mechanisms of obesity-linked cardiac dysfunction: An up-date on current knowledge. Cells 10(3): 629.
- Triposkiadis F, Xanthopoulos A, Starling RC, Iliodromitis E (2021) Obesity, inflammation, and heart failure: Links and misconceptions. Heart Fail Rev.
- Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, et al (2017) The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis 1863(5): 1106-1114.
- Zamora M, Villena JA (2019) Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int J Mol Sci 20(11): 2833.
- Mortuza R, Chakrabarti S (2014) Glucose-induced cell signaling in the pathogenesis of diabetic cardiomyopathy. Heart Fail Rev 19(1): 75-86.
- Vincent HK, Taylor AG (2006) Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes 30(3): 400-418.
- Bode D, Wen Y, Hegemann N, Primessnig U, Parwani A, et al (2020) Oxidative stress and inflammatory modulation of Ca2+ handling in metabolic HFpEF-related left atrial cardiomyopathy. Antioxidants 9(9): 860.
- Dhalla NS, Liu X, Panagia V, Takeda N (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40(2): 239-247.
- Tappia PS, Adameova A, Dhalla NS (2018) Attenuation of diabetes-induced cardiac and subcellular defects by sulphur-containing amino acids. Curr Med Chem 25(3): 336-345.
- Adameova A, Xu YJ, Duhamel TA, Tappia PS, Shan L, et al (2009) Anti-atherosclerotic molecules targeting oxidative stress and inflammation. Curr Pharm Des 15(27): 3094-3107.
- Al-Goblan AS, Al-Alfi MA, Khan MZ (2014) Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 7: 587-591.
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1): 207-258.
- Bahiru E, Hsiao R, Phillipson D, Watson KE (2021) Mechanisms and treatment of dyslipidemia in diabetes. Curr Cardiol Rep 23(4): 26.
- Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL (2021) Global epidemiology of dyslipidemia. Nat Rev Cardiol.
- Nakamura M, Sadoshima J (2020) Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol. 598(14): 2977-2993.
- Guzzardi MA, Iozzo P (2011) Fatty heart, cardiac damage, and inflammation. Rev Diabet Stud 8(3): 403-417.
- D Sousa K, Nzirorera C, Kienesberger PC (2016) Lipid metabolism and signaling in cardiac lipotoxicity. Biochim. Biophys. Acta 1861(10): 1513-1524.
- Li SJ, Wu TW, Chien MJ, Mersmann HJ, Chen CY (2019) Involvement of pericardial adipose tissue in cardiac fibrosis of dietary-induced obese minipegs-role of mitochondrial function. Biochim Biophys Acta Mol Cell Biol Lipids 1864(7): 957-965.
- Cappellari GG, Aleksova A, Ferro MD, Cannatà A, Semolic A, et al (2020) Preserved skeletal muscle mitochondrial function, redox state, inflammation and mass in obese mice with chronic heart failure. Nutrients 12(11): 3393.
- Dhalla NS, Ganguly, PK, Bhullar SK, Tappia PS (2019) Role of catecholamines in the pathogenesis of diabetic cardiomyopathy. Can J Physiol Pharmacol 97(9): 815-819.
- Carnagarin R, Lambert GW, Kiuchi MG, Nolde JM, Matthews VB, et al (2019) Effects of sympathetic modulation in metabolic disease. Ann NY Acad Sci 1454(1): 80-89.
- Lustig RH (2008) Which comes first? The obesity or the insulin? The behavior or the biochemistry. J Peds 152(5): 601-602.
- Ramjiawan M, Tappia PS, Dhalla NS. (2021) Hypothalamus-mediated actions in the genesis of obesity. In: Cellular and Biochemical Mechanisms of Obesity. Springer Nature, Switzerland.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




