
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Mini Review(ISSN: 2638-5910) 
Management of Postprandial Blood Glucose in Diabetes Mellitus Volume 1 - Issue 5
Poondy Gopalratnam Raman*
- MGM Medical College, 72, Dhar Kothi, Indore, India
Received: December 15, 2018; Published: December 19, 2018
Corresponding author: Poondy Gopalratnam Raman, MGM Medical College, 72, Dhar Kothi, Indore, India
DOI: 10.32474/ADO.2018.01.000124
Abstract
Patients with type-2 diabetes have more than half the day in the post meal state. Elevation in post meal plasma glucose is due to loss of first phase insulin secretion, decreased insulin sensitivity in peripheral tissues and consequent decreased suppression of hepatic glucose output after meals due to insulin deficiency. Elevated or exaggerated post meal response is directly responsible for endothelial dysfunction and pro-atherogenic states. Post-meal hyperglycemia is a prominent early defect in type-2 diabetes patients.
Keywords: Post prandial blood glucose; Insulin resistance; HbA1c level; Hyperglycemia; Drugs reducing PPBS
Introduction
80% of total caloric intake is carbohydrate rich in our country. The higher glucose load in the diets is responsible for an exaggerated prandial glycemic response. This in turn leads to a higher lipaemic peak which has a direct correlation with cardiovascular disease. Even before diagnosis of clinical diabetes, these metabolic abnormalities are first evident as elevation in post- meal plasma glucose. HbA1c levels reflect overall glycemic exposure and are determined by both fasting and PPG exposure. In a study patient who achieved fasting plasma glucose levels of < 100 mg/ dl, 64% achieved HbA1c level of <7% and of patients who achieved PPG levels < 140 mg/dl, 94% achieved an HbA1c < 7%. In diabetic patients with HbA1c levels between 6.8 to 8.9, PPG contributed to almost 70% of the HbA1c level.
Effect of Post Prandial Blood Glucose
Post prandial blood glucose has been linked to diabetic complications like cardiovascular problems. Achieving diet control in diabetic patients remains a significant challenge in our country. The value of good glycemic control by intensified insulin therapy in type 1 diabetes patients has been established by DCCT trial. Subsequent studies like UKPDS and Kumamoto confirm that the extent of benefit of tight control in type 2 patients also. In one study almost half the patients have post prandial values more than 70 mg over fasting values. 1/3 of patients had glucose excursion over 100 mg/dl. Elevated post prandial blood glucose with normal fasting blood glucose can cause complications like retinopathy. Some of the micro and macro vascular complications are seen even before the diagnosis of DM clinically. The Rancho Bernardo study [1] suggests that isolated post challenge hyperglycemia increase the risk of fatal CVD. Plasma glucose value more than 140 mg% at 2 hours after an OGTT defines postprandial hyperglycemia (PPHG). It includes all cases of IGT. Post prandial glucose reduction is seen with metformin, alpha glucosidase inhibitors, pioglitazone and DPP-4 inhibitors.
Causes of High Postprandial Blood Glucose are
a. Unusually high intake of carbohydrate diet.
b. OHA or insulin when given at pre-lunch or pre-breakfast time is not able to bring post lunch blood sugar but is controlling fasting blood glucose. Any attempt to increase the dose of medication in such situation results in 5.00 pm hypo glycaemia (Evening Hypo glycaemia) hence is not the preferred mode of treatment.
c. In Type 2 DM meal stimulated insulin secretion seems to be lost. This occurs even before overt DM develops. Normally after IV Glucose, insulin peaks within 10 minutes and second phase peaks after 20 minutes. In DM insulin in 1st phase is absent, and second stage secretion is blunted and delayed. Fasting hyper glycaemia occurs when a loss of approximately 75% in beta cells occur.
d. Due to b-cell dysfunction there is altered insulin pulsatility both in timing and amplitude, low insulin and high glucagon levels increase hepatic glucose output.
e. Insulin resistance aggravates the non-suppressibility of hepatic glucose output and also reduces peripheral glucose uptake.
f. There is defect in glut-2 and glut-4 transporter system
g. Kidney has a role in postprandial hyperglycemia
Patho phytologic mechanism of tissue damage due to post-meal hyperglycemia
Hyperglycemia Induced Mitochondrial Production Leads to
a. Increased flux in polyol pathway
b. Increased intracellular formation of advanced glycation endproducts
c. Protein kinase C activation
d. Increased flux through hexosamine pathway
How to Recognize Post-Meal Hyperglycemia?
a. Self-monitoring blood glucose is ideal for deducting and monitoring post-meal glucose profile
b. Continuous monitoring of blood glucose profile adds to identification of post-meal surges in apparently well controlled diabetes
c. Fructosamine and 1, 5 – anhydroglucitol measure short-term glucose exposure. They accurately reflect post-meal blood glucose value.
Glucose monitoring in type2 diabetes mellitus seems to be more complex than previously thought, because fasting glucose is a rather poor index of glucose levels throughout the day. HbA1c seems to provide poor information on postprandial glucose levels and it provides no information on glucose excursions with meals. Post-meal is one of the earliest defects in diabetes and is the predominant contributor to HbA1C at values below 8.4%
PPBG Elevations and DM
Glucose is a potent inducer of oxidative stress and induces free radical oxidation of low-density lipoprotein. This leads to increased vessel wall atherogenesis. Increased PPBG produces dyslipidemia, activates prothrombotic activity and reduces insulin sensitivity. All these factors lead to chronic complications of DM. In gestational diabetes normalizing postprandial glucose levels are associated with a better outcome of pregnancy. [2] There is epidemiologic evidence to show that postprandial hypoglycemia correlated directly with incidence of retinopathy, nephropathy, cerebro-vascular accidents and their outcome. The Decode study shows that meal time glucose spikes are independent risk factors for cardiovascular mortality. White Hall study [3] the mortality doubled in those with postprandial levels between 96 mg/dL and 146 mg/dL compared to those with <96 mg/dL. In the Islington Diabetes Survey [4] the prevalence of major CVD increased from 9% in those with normal glucose tolerance to 19% in those with IGT (Table 1) [5-19].
Table 1: Relationship of postprandial hyperglycemia to chronic complications of diabetes.
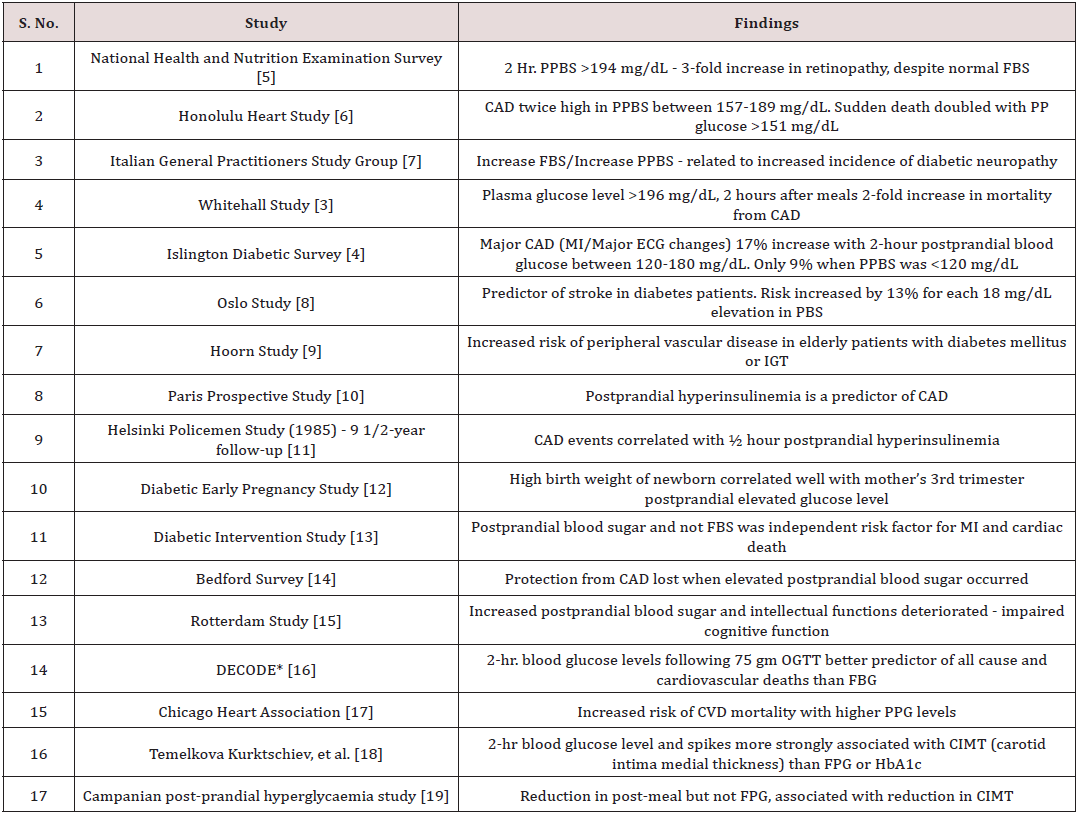
*DECODE- Diabetes epidemiology collaborative analysis of diagnostic criteria in Europe.
Management [20]
A drug should reduce rate of glucose entry, improve meal related insulin level and inhibit glucagon effect in order to be effective in controlling PPG.
a. Reduce carbohydrate intake at lunch. Diets with a low glycaemic index are beneficial like legumes, pasta and most fruits. Diets with high GI viz. potatoes, white and brown bread, rice are to be minimized. Sugars which are slowly digested and absorbed are less glycemic by nature viz. fructose and lactose.
b. Incretin Mimetics (Exenatide), GLP1 analogue (liraglutide), DPP-4 (sitagliptin and vildagliptin) and Amylin analogues can be used [21].
c. α-glucosidase inhibitors [21] is a complex oligosaccharide which acts on alpha-glucosidase in the brush border of the small intestine by competitively inhibiting α-glucosidase. This inhibition delays digestion of complex carbohydrates in the upper GI and retards absorption of glucose and blunts postprandial hyperglycemia. Voglibose is approximately 20-30 folds more potent inhibitor of small intestinal disaccharides as compared with Acarbose. It has less gastric side effects. Reduces oxidative stress generation and reduces PPG and lipids in obese type-2 diabetes. Side effects are flatulence, abdominal distension, borborgymi and diarrhoea. The drug lowers insulin raises in post prandial hyper glycaemia, no weight gain occurs with the therapy and there are no hypoglycemic episodes when given as monotherapy. Available as 50mgm tablets, maximum is 100mgm/day. If hypo glycaemia develops in combination therapy, glucose should be used and not sucrose or table sugar.
d. Insulin analogue and short-acting regular insulin are useful. Lispro [22] insulin is an insulin analogue, has proline from position B28 to B29, thus Lispro hexamers dissociate more readily than regular human insulin hexamers into monomers. It is indicated for reduction of pre-prandial and post-prandial blood sugar. As it is a short acting insulin and it has to be used in conjunction with longer acting human insulin. It can- be taken with meals. It has rapid onset action and shorter duration of action than regular human insulin. If a person is taking 30/70 mixture of injection Human Mix tard insulin it can be changed to 50/50 i.e. containing equal parts of short acting and intermediate acting insulin.
e. Long acting basal insulin (Glargine) can also be used in order to bring fasting hyperglycemia and once fasting hyperglycemia is controlled, postprandial hyperglycemia may also be controlled to some extent.
f. Metformin 0.5 gm twice before meals can be tried to increase insulin sensitivity. Maximum dose of 3 gm can be tried if there is no GI intolerance.
g. Nateglinide and Repaglinide are non sulphonylurea drugs when taken before meals effectively reduce post meal hyper glycaemia. They are short acting anti-diabetic drugs.
Conclusion
Persistent post prandial hyper glycaemia in a diabetic is not desirable. It will lead to long term macro-vascular and micro vascular complications. Every effort should be made to bring it down to normal acceptable level. Current modalities of therapy to bring down prandial hyper glycaemia are briefly reviewed.
References
- Barret Connr EL, Cohan BA, Wingart BL, Edelstein SL (1991) The Rancho Bernardo Study. JAMA 205: 627.
- Jovanovic Peterson L, Peterson CM, Reed GF, et al. (1991) Maternal postprandial glucose levels and infant birth weight: The Diabetes in Early Pregnancy Study. Am J Obstet Gynaecol 164: 103-111.
- Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H (1980) Coronary-heart disease risk and impaired glucose tolerance, the Whitehall Study. Lancet 1(8183): 1373-1376.
- Jackson CA, Yudkin JS, Forrest RD (1992) A comparison of the relationships of the glucose tolerance test and the glycated haemoglobin assay with diabetic vascular disease in the community. The Islington Diabetes Survey. Diabetes Res Clin Pract 17(2): 111-123.
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, et al. (1998) Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes care 21(4): 518-524.
- Donahue RP, Abbott RD, Reed DM, Yano K (1987) Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes 36(6): 689-692.
- Beghi E, Monticelli ML (1997) Diabetic polyneuropathy in the elderly. Prevalence and risk factors in two geographic areas of Italy. Italian General Practitioner Study Group (IGPSG). Acta Neurol Scan 96(4): 223- 228.
- Haheim LL, Holme I, Hjermann I, Leren P (1995) Nonfasting serum glucose and the risk of fatal stroke in diabetic and nondiabetic subjects. 18-year follow-up of the Oslo Study. Stroke 26(5): 774-777.
- Beks PJ, Mackaay AJC, de Neeling JN, de Vries H, Bouter LM, et al. (1995) Peripheral arterial disease in relation to glycemic level in an elderly Caucasian population: The Hoorn Study. Diabetologia 38(1): 86-96.
- Fontbonne A, Charles MA, Thibult NA, Richard JL, Claude JR, et al. (1991) Hyper insulinaemia as a predictor of coronary heart disease mortality in a healthy population: the Paris Prospective Study, 15-year follow-up. Diabetologia. 34(5): 356-361.
- Pyorala K, Savolainen E, Kaukola S, Haapakoski J (1985) Plasma insulin as coronary heart disease risk factor: relationship to other risk factors and predictive value during 9-1/2-year follow-up of the Helsinki Policemen Study population. Acta Med Scand 701(Suppl): 38-52.
- Radhika Jindal, Nitin Gupta, Mohammad Asim Siddiqui, Subhash Kumar Wangnoo (2013) Post-prandial hyperglycaemia.14(3): 242-246.
- Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, et al. (1996) Risk factors for myocardial infarction and death in newly detected NIDDM: The Diabetes Intervention Study, 11-year follow-up. Diabetologia 39(12): 1577-1583.
- Jarrett RJ, McCartney P, Keen H (1982) The Bedford Survey: Ten-year mortality rates in newly diagnosed diabetics, borderline diabetics, and normoglycemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia 22(2): 79-84.
- Stolk RP, Breteler MMB, Ott A, Pols HA, Lamberts SW, et al. (1997) Insulin and cognitive function in an elderly population, the Rotterdam Study. Diabetes Care 20(5): 792-795.
- Balkau B (2000) The DECODE study. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Diabetes Metabolism 26(4):2 82-286.
- Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, et al. (1997) Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men: the Chicago Heart Association Detection Project in Industry study. Diabetes Care 20(2):163-169.
- Hanenfeld M, Koehler C, Schaper F, Fuecker K, Henkel E, et al. (1999) Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis 144(1): 229-235.
- Esposito K, Giugliano D, Nappo F, Marfella R (2004) Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 110(2): 214-219.
- International Diabetes Federation (2011) Guideline for Management of Post Meal Glucose in Diabetes.
- Sephen P Clissol, Clive Edward (1988) Acarbose. A review of its pharmacodynamics and pharmacokinetic properties and therapeutic potential. Drugs 35(3): 214-243.
- Anderson, JH, Brunelle RL, Keohane P, Koivisto VA, Trautmann ME, et al. (1997) Mealtime treatment with insulin analogue improves Post Prandial Hyperglycaemia and Hypoglycaemia in NIDDM patients. Multicenter Insulin Lispro Study Group. Arch Internal Medicine 157(11): 1249-1255.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














