
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
Evaluation of the Prevalence of Gestational Diabetes Using Fasting Blood Glucose and Glycated Heamoglobin in Yenagoa Metropolis Volume 1 - Issue 1
Gborienemi Simeon George*and Duoboye Stephen Enetim
- Department of Medical Laboratory Science, Niger Delta University, Nigeria
Received: May 17, 2018; Published: May 22, 2018
Corresponding author:George Gborienemi Simeon (Ph.D), Department of Medical Laboratory Science, Niger Delta University, Wilberforce Island, Amassoma, Bayelsa State, Nigeria
DOI: 10.32474/ADO.2018.01.000104
Abstract
There is understanding that most pregnant women with gestational diabetes mellitus stands the risk of having adverse obstetric and perinatal outcome. The need for an early detection and effective management of this problem with a view to ensure better maternal and fetal protection was the driving thrust for this study. Through the application of spectrophotometric methods employing enzymatic and non-enzymatic systems, the concentration of fasting blood glucose (FBG) and glycated Haemoglobin (HbA1c) were determined in 2nd and 3rd trimester pregnant women and compared with non-pregnant women. Data were analysed using student’s t-test with the aid of Graph pad Prism (R) software version 6.10 at p<0.05 values considered statistically significant. Result reveals that 11% of pregnant women investigated had gestational diabetes mellitus in Yenagoa metropolis. Our findings elucidate the danger of gestational diabetes mellitus, its prevalence and the need to allow for effective proactive intervention program regarding screening and management and in addition highlight areas requiring further research.
Keywords: Gestation; Diabetes mellitus; Fasting blood glucose; Glycated hemoglobin
Abbrevations: FBG: Fasting Blood Glucose; GDM: Gestational Diabetes Mellitus; RBC: Red Blood Cells; EDTA: Ethylene Diamine Tetrachloro Acetic Acid
Introduction
Diabetes mellitus is a group of metabolic disease conditions that have significantly contributed to increasing health burden and financial problem of many countries worldwide. Although the prevalence and screening methods for the two major clinical subgroups type 1 and type 2 diabetes are well researched and to a large extent, the mechanism are understood, other subgroups of this disease notably gestational diabetes mellitus (GDM) are less established. Gestational diabetes mellitus is defined as glucose intolerance of variable degree with onset or first recognition during pregnancy which as a concept, existed since 1964 [1,2] established that GDM occurs in about 2-10% of all pregnancies and the condition may improve or disappear after delivery. Studies of [3] have observed that GDM is estimated to affect 1% to 14% of pregnancies in the United States annually, depending on the population studied and the diagnosis test method used. It has been shown in [4] that gestational diabetes mellitus prevalence has been steadily increasing with the rise of obesity and type 2diabetes. Both birth certificates and pregnancy risk assessment monitoring system which include questionnaire completed by others can provide population based prevalence estimate of gestational diabetes mellitus.
Studies indicates that whereas specificity for gestational diabetes mellitus is high, on the birth certificate sensitivity is as low as 48%, thus gestational diabetes prevalence obtained from the birth certificate alone is likely underestimated. In contrast, pregnancy risk assessment monitoring system may overestimate GDM prevalence. The exact mechanism underlying GDM is still not clear. The hallmark of GDM however, is increased insulin resistance. As elucidated [5] pregnancy hormones and other factors are thought to interfere with the action of insulin as it readily binds to the insulin receptor. Insulin resistance is known to be a normal phenomenon which sparks up in the second trimester of pregnancy and progresses further thereafter to levels seen in non-pregnant patients with type 2 diabetes. It is unclear why some patients are unable to balance needs and develop GDM but it has been shown by [6] and [7] that autoimmunity, single gene mutations, obesity and other mechanism cannot be ruled out. Studies by [8-10] have ascribed this problem to loss of insulin producing beta cells of the islet of Langer hans. It had earlier been shown [11] that women with gestational diabetes are at high risk for pregnancy and delivery complications including infant macrosomia, neonatal hypoglycemia and cesarean delivery. Earlier work by [12] revealed that women who are affected by GDM have more increased risk of developing type 2 diabetes 5 to 10 years after delivery. It has also been shown by [13] that children born to mothers with gestational diabetes are also more likely to develop impaired glucose tolerance. It has been shown by [14] that glycated hemoglobin is a form of hemoglobin that is measured primarily to identify the three month average plasma glucose concentration. The test is limited to three months average plasma glucose concentration. This is because the life span of a red blood cell is four months about (120 days). However, since red blood cells (RBcs), do not all undergo lysis at the same time, glycated hemoglobin is taken as a limited measure of 3 months. Glycated hemoglobin is a measure of the beta-N-1-deoxyfructosyl component of hemoglobin. Previous report shows that normal levels of glucose produce a normal amount of glycated hemoglobin [15]. When blood glucose is high, glucose molecules attach to the hemoglobin in red cells. The longer hyperglycemia persists, the more glucose bind to the hemoglobin in the red blood cells and the higher the glycated hemoglobin. Once hemoglobin molecule is glycated, it remains that way. A build up of glycated hemoglobin within the red cell therefore reflect the average level of glucose to which cells have been exposed during its life-cycle.
Measuring glycated hemoglobin assesses the effectiveness of therapy by monitoring long-term glucose regulation. It has been shown that measurement of glycated hemoglobin is effective in monitoring long-term glucose control in people with diabetes mellitus [16]. It provides a retrospective index of the integrated plasma glucose values over an extended period of time and is not subject to the wide fluctuations observed when assaying blood glucose concentration. Our findings and implications for life of gestational diabetes mellitus patients are encapsulated in this work.
Material and Methods
Location/subjects
The study was conducted in Yenagoa, Bayelsa State, Nigeria among pregnant women in second and third trimester of pregnancy attending clinic in Diette Koki Hospital and Niger Delta University Teaching Hospital. The study population consisted of 100 pregnant women and 100 non-pregnant women that served as control.Ethical approval for this human study was obtained both from the Niger Delta University and the Diette Koki Hospital. Patients consent were sought for and agreed before sample collection commenced.
Samples
5.0ml of venous blood was collected from fasting subjects at 8.30am and the samples were ali quoted to fluoride bottle and ethylene diamine tetrachloro acetic acid (EDTA) containers. Sample in fluoride containers were spun to obtain serum for glucose determination. Samples in EDTA bottle were used for glycated hemoglobin (HbA1c) determination.
Analytical method
Glucose was determined by the glucose-oxidase-peroxidase method (Randox Product, UK). The glucose in sample was catalysed oxidatively by the glucose oxidase and converted to hydrogen peroxide and gluconic acid. The hydrogen peroxide was broken down by peroxidase and oxygen released reacts with 4-aminophenozone and phenol to give pink color whose absorbance was measured at 540nm with the use of spectrophotometer 22D+ (product of UNISCOPE, England). Glycated hemoglobin was determined nonenzymatically by first cleaving hemoglobin into peptides by the enzymes endoproteinase Glu-c, and in a second step by the glycated and non-glycated N-terminal hexa peptides of the ß–chain obtained were separated and quantified by ion-exchange high performance liquid chromatography (HPLC-Esi/ms) with UV-detection. Principle depend on the fact that a non-enzymatic reaction occurs between glucose and the N-terminal of the Beta-chain forming a Schiff base which is itself converted to 1-deoxyfructose an Amadori rearrangement. The longer hyperglycemia occurs in blood, the more glucose binds to hemoglobin and the higher the glycated hemoglobin concentration.
Statistical analysis
Data were analysed using student’s t-test with the aid of Graph pad Prism (R) software version 6.01 p values of <0.05 were considered statistically significant.
Result
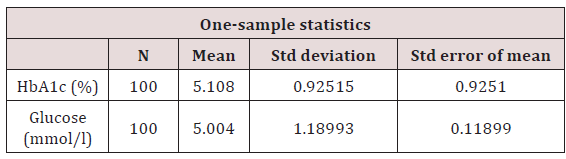
The concentration of glucose and glycated hemoglobin in pregnant and non-pregnant subjects is shown in tables below. Table 1 is a one sample statistics for the pregnant women showing their glucose level and glycated hemoglobin. In Table 2 below show a comparison plot of glucose concentration in pregnant and nonpregnant. In Table 3 below a one sample statistics is shown for glycated hemoglobin concentration in pregnant and non-pregnant (Table 4) and (Figure 1).
Table 1: Glucose and HbA1c concentration.
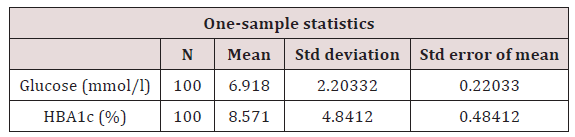
t=31.398, p=0.0001. P is significant at <0.05. Values on table suggest a relationship at p = 0.0001
Figure 1: Is a scatter plot showing the relationship of glucose with glycated hemoglobin. The plot shows that both parameters correlate positively.
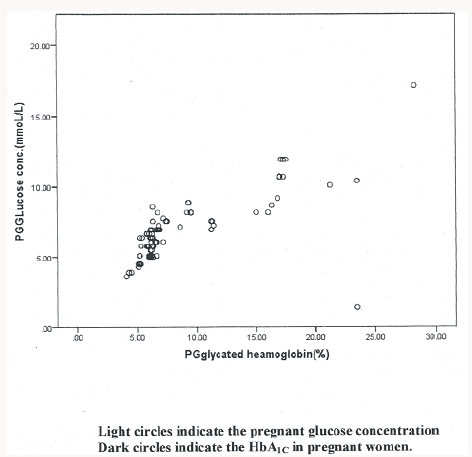
Table 2: Glucose concentration in pregnant and non-pregnant subjects.
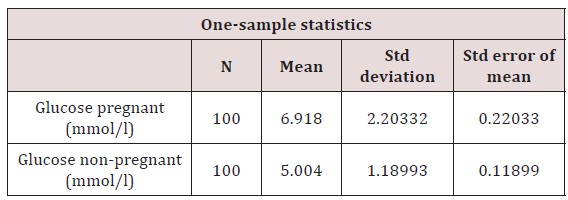
t=31.398, p=0.0001. P is significant at <0.05. Values on table suggest a relationship at p = 0.0001
Table 3: Glycated Haemoglobin in pregnant and non-pregnant.
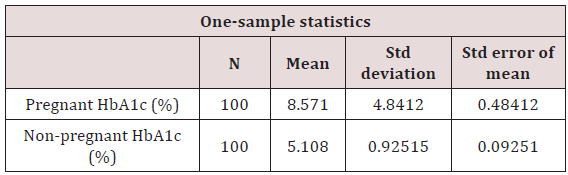
t=17.71, P=0.0001. P is significant at <0.05. Values on table suggest a relationship at p = 0.0001
Table 4: Glucose and glycated Haemoglobin concentration in non-pregnant.
t=55.21. P=0.0001. P is significant at <0.05. Values on table suggest a relationship at p = 0.0001
Discussion
Concern for the health of the pregnant woman and he unborn child have driven the heightened interest in the investigation of gestational diabetes mellitus. The diagnostic criteria for gestational diabetes are varied and include screening of high risk patients, strong history of diabetes, and history of abnormal glucose metabolism, presence of glucosuria, diagnosis of polycystic ovarian syndrome, overweight and being a member of an ethnic/racial group with a high prevalence of diabetes mellitus. Pregnancy is known to induce a state of insulin resistance. This condition is usually elucidated especially at late pregnancy due to lowering of the renal threshold. Under this condition there is an increased demand on ß-cells function which may reveal sub-clinical aberrations in carbohydrate homeostasis which may not be normally apparent in a non-pregnant woman.
The present study which is the first of its kind undertaken in this part of the country showed the prevalence of GDM as 11%. It has however been reported variably from 1.4-14% worldwide and differently among racial and ethnic groups [3]. It was revealed that values obtained also depend on the population studied and the diagnostic test used [2]. Studies by [17] revealed that gestational diabetes mellitus rate differ by state with the greatest variation attributable to difference in obesity. Obesity is known to be associated with gestational diabetes and could be prevented if we reduce the risk of overweight women. Preventing obesity is a key component of good women care regardless of pregnancy intention.
We have used only those pregnant women whose glucose was close or above the threshold of 10.0mmol/l in this study in determining the prevalence on account of the criticality of the threshold value. The inclusion of the glycated hemoglobin in this study was intended to show how they correlate in GDM in consideration of the complications in diabetes. Earlier result [18] had epitomized this epidemiological profile of diabetics in pregnancy. The study by [19] also brought to the fore the need for care of diabetic mothers. It has also been recognized that most women with GDM revert back to normal glucose metabolism after delivery of their babies. It has however been observed that they stand the risk of developing type 2 diabetes later in life as are their offspring. Other common maternal complications include hypertension, vaginal candidiasis and abruption placenta with the possibility of macrosomia and stillbirths occurring in the fetus.
Our findings in this work are to a large extent at tandem with those found in the literature both at national and international level. The inference to be drawn from this study is that Yenagoa metropolis inspite of its differential ethnicity being a state capital, living standard, and variation in food is not significantly different from the diabetic incursion world over.
In conclusion, this work buttresses the need to initiate effective policy guidelines with intervention programmes to systematically structure and strengthen care for the pregnant woman and the fetus.
Acknowledgement
We acknowledge the generosity of Geo tech Medical Laboratory, Yenagoa and the Niger Delta University Laboratory where the tests were carried out having provided the needed equipment and reagents support. The co-operation of staff of these establishments is hereby highly acknowledged.
References
- Metzger BE, Coustan DM (2008) Organising committee: Summary and recommendations of the fourth international workshop conference on Gestational diabetes mellitus. Diabetes Care 21 (Suppl 2): B161-B167.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, et al. (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet 380: 219- 229.
- Hunt KJ, Schuller KL (2007) The increasing prevalence of diabetes in pregnancy. Obstetrics and Gynecology clinics of North America 34(2): 173-99.
- Delvin HM, Desai J, Walaszek A (2009) Reviewing performance of birth certificate and hospital discharge data to identity births complicated by maternal diabetes. Maternal child health journal 13(5): 660-666.
- Macaulay S, Dunger DB, Norris SA (2014) Gestational diabetes mellitus in Africa: A systemic review. PLOS ONE 9(6): e97871.
- Nilofer AR, Raju VS, Dakshayini BR, Zaki SA (2012) Screening in highrisk group of gestational diabetes mellitus with its maternal and fetal outcome. Indian J Endocrinal metab 16(Suppl 1): 74-78.
- Madi JM, Viecceli C, Barazzetti DO, Pavan G, Triches CB, et al. (2011) Gestational diabetes and perinatal outcomes: A case control study. Journal of medicine and medical science 2(8): 1022-1027.
- Vasudeuan DM, Sreeku MS, Kannan V (2011) Textbook of biochemistry for medical students 6th Edition, Jitendar P Vij. New Delhi India 274-291.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152979/
- Tuomi T, Santoro N, Caprio S, Cai M, Weng J et al. (2014) The many faces of diabetes: A disease with increasing heterogeneity. Lancet 383(9922): 1084-1094.
- Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode M et al. (2012) Gestational diabetes and pregnancy outcomes-A systematic review of the World Health Organisation (WHO) and the International Association of Diabetes in Pregnancy Study Groups Diagnostic Criteria. Biomedical central pregnancy child birth 12(1): 23.
- Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: Systemic review and meta-analysis. Lancet 373(9677): 1773-1739.
- Dabelea D, Mayer-Davis EI, Lamichhane AP, Dagostino RB, Liese AD (2008) Association of Intrauterine Exposure to Maternal Diabetes and Obesity with type 2 diabetes in youth: The search case-control study. Diabetes Care 31(7): 1422-1426.
- Miedema K (2005) Standardization of HbA1c and optinal range of monitoring. Scandinavian Journal of Clinical and Laboratory Investigation (Suppl 240): 61-72.
- Larsen ML, Horder M, Mogensen EF (1990) Effect of long term monitoring of glycosylated haemoglobin levels in insulin dependent diabetes mellitus. New England Journal Medicine 323 (15): 1021-1025.
- Carl AB, Edward R, Ashwood, David EB (2008) Tietz fundamentals of clinical chemistry 6th edition, Elsevier. New Delhi 373-401.
- Bardenheier BH, Elixhanser A, Imperatere G, Delvin HM et al. (2013) Variation in prevalence of gestational diabetes mellitus in prevalence of gestational diabetes mellitus among hospital discharges for obstetric deliver across 23 states in the United State. Diabetes Care 36(5): 1209- 1214.
- Gomez HL, Martinez ML, Rodriguez ZM (2011) Clinical and epidemiological profile of diabetes in pregnancy, Isle youth, 2008. MEDICC Rev 13(1): 29-34.
- Opara PI, Jaja T, and Onubogu UC (2010) Morbidity and mortality amongst infants of diabetic mothers admitted into a special care unit in Port Harcourt, Nigeria. Hal J Pediator 36(1): 77.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...








