
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
Epicardial Adipose Tissue Inflammation and Fibrosis: Relationships with Coronary Artery Diseases Volume 3 - Issue 4
Rizzatti Vanni1, Fantin Francesco2, Mazzali Gloria1, Zoico Elena2, Rossi Andrea1, Faggian Giuseppe1, Onorati Francesco1, Santini Francesco3 and Zamboni Mauro1*
- 1Department of Surgery, Dentistry, Maternity and Infant, Section of Geriatrics, University of Verona, Italy;
- 2Department of Medicine, Section of Geriatrics, University of Verona, Italy
- 3Department of Surgery, Dentistry, Maternity and Infant Section of Cardiac Surgery, University of Verona, Italy
- 4Division of Cardiac Surgery, University of Genova, Italy
Received:May 28, 2021; Published: June 4, 2021
Corresponding author: Mauro Zamboni, Department of Surgery, Clinica Geriatrica, Ospedale Maggiore, Healthy Aging Center, University of Verona, Verona, Italy
DOI: 10.32474/ADO.2021.03.000166
Abstract
Aims: Accumulation of epicardial adipose tissue (EAT) is associated with severity and progression of coronary artery disease (CAD). The aim of this study was to compare EAT fibrosis, inflammation, Hypoxia-inducible factor 1-alpha (HIF1-α) and caveolin-1 (CAV-1) between subjects with and without CAD.
Methods and Results: Body mass index (BMI), waist circumference (WC), glucose, insulin, homeostasis model assessment index, serum leptin and adiponectin were evaluated in EAT of patients with and without CAD undergoing elective surgery. Biopsies were collected from EAT. Immunohistochemistry for macrophages CD68, CD11c, CD163, HIF1-α and CAV-1 was performed and Masson Trichrome staining to define the degree of fibrosis. A total of 22 male patients (age range from 51-80 years), were studied: 12 CAD and 10 non-CAD undergoing elective surgery.
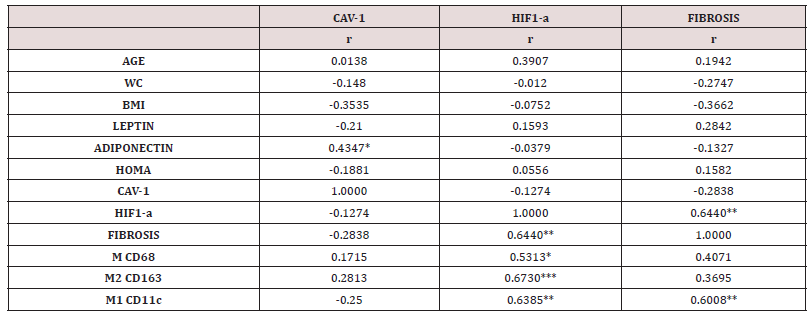
Fibrosis (1.29 ± 0.71 vs 0.54 ± 0.40, p<0.01), HIF1-α (2.39 ± 1.64 vs 1.01 ± 0.94, p<0.05), number of total CD68 (74.25 ± 49.39 vs 37.33 ± 18.28, p<0.05), CD163 (57.75 ± 38.23 vs 31.11 ± 18.54, p<0.05) and CD11c (6.67 ± 4.73 vs 2.05 ± 1.13, p<0.01) were higher in CAD than in non-CAD patients, whilst serum adiponectin (8.25 ± 7.52 vs 15.06 ± 11.11, p<0.05), and CAV-1 (0.0790 ± 0.0268 vs 0.0987 ± 0.0164, p<0.05) significantly lower. In 4 patients with CAD, but in none in those without, macrophages aggregated in crown like structure were found. Fibrosis correlated with HIF1-α (r= 0.644, p < 0.01) and M1 pro-inflammatory CD11c (+) macrophages (r=0.601, p<0.01); HIF1-α with total, M1 pro-inflammatory CD11c (+) and M2 anti-inflammatory CD163 (+) macrophages (r=0.5313, p<0.05; r=0.6385, p<0.01; r=0.6730, p<0.001, respectively); Caveolin-1 positively correlated with serum adiponectin (r=0.4347, p<0.05).
Conclusions: CAD patients displays a dysfunctional EAT characterized by greater inflammation, fibrosis, HIF1-α and lower CAV. Our results seem to suggest that adiponectin may decline CAD risk even by determining an increase of CAV-1.
Keywords: Epicardial Adipose Tissue; HIF‐1α; Fibrosis; Inflammation; Coronary Artery Disease
Abbreviations: BMI, Body Mass Index; WC, Waist Circumference; HOMA, Homeostasis Model Assessment; CAV-1, caveolin 1; ADIP DIAMETER, diameter of adipocytes; HIF1-α, number of adipocytes nuclei positive for Hypoxia-inducible factor 1-alpha M CD68, Total Macrophages; M2 CD163, Anti-inflammatory Macrophages; M1 CD11c, Proinflammatory Macrophages *p<0.05, **p<0.01, ***p<0.01.
Introduction
Coronary artery disease (CAD) is the most common cardiovascular disease and the leading cause of mortality in developed Countries [1,2]. Accumulation of epicardial adipose tissue (EAT) is associated with severity and progression of CAD, both in obese and non-obese people. Studies support the hypothesis that inflammation of EAT can influence structure and function of coronary artery. EAT may also mediate the influence of systemic inflammation on coronary arteries. EAT is considered to be part of the visceral fat, located between the surface of the myocardium and the pericardium, which covers about 80% of the heart surface. EAT shows anatomical continuity with the myocardium due to the absence of a layer that separates the two different tissues [3]. The closeness and the absence of anatomical boundaries between EAT and heart determines that mediators such as adipokines and cytokines released by EAT have paracrine effects on the myocardium. EAT plays an important role in cardiovascular diseases such as coronary artery disease (CAD), as well in atrial fibrillation and heart failure [4,5].
Higher values of interleukin 6 and tumor necrosis factor alpha as well lower of adiponectin have been found in the EAT of CAD patients compared to that of subjects without [6,7]. It has also been shown that EAT of CAD patients displays an infiltrate of inflammatory cells such as macrophages and lymphocytes associated with an increase in inflammatory cytokines and a decrease in adiponectin gene expressions [7]. Greater infiltration of M1 pro-inflammatory macrophages has been found in EAT of CAD patients than in that of those without [8]. Together with inflammation greater fibrosis has been shown in EAT of patients with atrial fibrillation [9]. Hypoxia is a common feature in cardiovascular disorders such as atherosclerosis and heart failure. Hypoxia inducible factor 1-alpha (HIF1-α) transcription factor, mediator of cellular response to hypoxia, has a key role in the initiation or regulation of inflammation and tissue remodeling, in particular in the activation of M1 proinflammatory macrophages [10] and in the genesis of pathological fibrosis [11], which are common processes related to heart failure [12].
Studies have shown that Caveolae are essential in cardiac protection from myocardial ischemia / reperfusion injury [13]; Caveolin-1 (CAV-1) knockout mice exhibited dilated cardiomyopathy and right ventricular hypertrophy compared with wild-type mice [14]. Caveolae are a subset of lipid rafts, flask-shaped smooth invaginations of the plasma membrane rich in cholesterol and saturated linear chain fatty acids, abundantly expressed in adipocytes [15,16,17]. CAV1 and cavin1 are the main components required for the formation of the caveolae; CAV-1 and cavin-1 interact with the signaling complexes in the caveolae and regulate the transmission of signals through the membrane, including those of the cardiovascular system [16,18,19]. The aim of this study was to compare markers of dysfunctional EAT in patients with and without CAD. As markers of dysfunctional EAT increase in macrophages infiltration, fibrosis, HIF1-α as well as decline in CAV-1 were chosen
Material and Methods
A total of 22 male subjects, aged between 51 and 80 years (mean 67.86 ± 7.75 years), with BMI ranging from 23.18 to 32.87 kg/m2 (mean 27.57 ± 2.69 kg/m2), undergoing elective cardiac surgery either for coronary bypass grafting (CAD, n = 12) or valve replacement (non-CAD, n = 10) were included in the study. Clinical records were collected in all the subjects. Six patients had type 2 diabetes mellitus (DM) in the CAD group, 3 subjects in the non-CAD group. Patients with liver, kidney diseases or neoplastic diseases were excluded from the protocol study, as well as subjects with weight loss greater than 5% in the month preceding the evaluation, patients on steroid or on immunosuppressive therapy in the previous six months, patients with renal insufficiency with creatinine values > 1.5 mg / dl, poorly controlled thyroid disease, patients on hormonal therapy (except for the thyroid) and patients with chronic inflammatory diseases. Written informed consent was obtained from each participant and the study was approved by the Ethics Committee of the University of Verona (Italy).
Anthropometric Variables
Each patient underwent measurement of body weight with an approximation of 0.1 kg (Salus Scale, Milan, Italy) and to height measurement using a stadiometer, with an approximation of 0.5 cm. (Salus Stadiometer, Milan, Italy). BMI was computed as weight in kilograms divided by height in square meters (kg/m²). The measurement of WC was obtained with the patient standing upright, using a tape measure. The WC was assessed as the minimum abdominal circumference between the xiphoid process and the navel.
Blood Collection
Venous blood samples for all metabolic assessments were obtained after overnight fasting. Plasma glucose was measured with a glucose analyzer (Beckman Instruments Inc, Palo Alto, CA). The intra-assay CV was 1.5%. Plasma insulin was evaluated using a direct chemiluminescent-based two-site sandwich immunoassay (ADVIA Centaur Insulin assay; Siemens, Erlangen, Germany). The sensitivity of the assay was 0.5mU/l. We used the homeostatic model assessment (HOMA) method as an indirect index of insulin resistance, calculated as the product of the fasting plasma insulin level (mU/l) and the fasting plasma glucose level (mmol/l), divided by the constant 22.5. Serum leptin and adiponectin were measured using specific enzyme-linked immunoassay kits (respectively from DBC-Diagnostic Biochem Canada, London, ON, Canada and B-Bridge, Cupertino, CA,USA). The sensitivity of the assays was 0.5ng/ml for leptin and 0.02ng/ml for adiponectin.
Epicardial adipose tissue Collection
In the operating room, epicardial adipose tissue (EAT) specimens were obtained after median sternotomy and heparin administration prior to initiating cardio-pulmonary bypass, approximately 1 hour within induction of anesthesia. EAT fragments (approximately 1 gram) were collected during the atrial appendage cannulation procedure for extracorporeal circulation. EAT was then stored for immunohistochemistry (IHC) assay.
Immunohistochemistry
Freshly isolated EAT was fixed by immersion in 4% paraformaldehyde in 0.1M phosphate buffer, pH7.4 overnight at 4°C, and then dehydrated, cleared and paraffin embedded. Five micrometer thick serial sections were obtained and stained by hematoxylin and eosin (H/E) to assess morphology prior to immunohistochemical processing.
The following primary antibodies were used for the IHC analysis: mouse anti-human CD68 (ready to use; Thermo Fisher Scientific), mouse anti-human CD163 (1:300; Thermo Fisher Scientific), rabbit anti-human CD11c (3μg/ml; Thermo Fisher Scientific), rabbit antihuman HIF1-α (1:400; DBA Italia), rabbit anti-human Caveolin-1 (1:400, Cell Signaling). Secondary antibodies were as follows: Signal Stain Boost IHC detection reagent HRP Rabbit (ready to use; Cell Signaling) and Signal Stain Boost IHC detection reagent HRP Mouse (ready to use; Cell Signaling). A negative control was conducted by using a secondary antibody alone. Diaminobenzidine hydrochloride (DAB) substrate kit for peroxidase was used for IHC (Vector Laboratories).
Briefly, sections were dewaxed and subjected to antigen heat retrieval. Endogenous peroxidase activity and non-specific binding were blocked by incubation with 3% hydrogen peroxide and non-immune serum, respectively. Slides were then incubated sequentially with primary rabbit antibody for 16hr at 4°C or with primary mouse antibody for 1hr at 20°C, followed by incubation with the secondary antibody for 30 minutes at 20°C. DAB was used as the chromogen to visualize peroxidase activity. Sections were counterstained with hematoxylin, assemble with Entellan and overlaid with coverslips.
Masson Trichrome Staining for Interstitial Fibrosis Evaluation
EAT were stained with Masson’s trichrome to visualize interstitial fibrosis, according to the manufacturer’s protocol (Bio-Optica, Milano, Italy). Briefly, sections were deparaffined, rehydrated, stained with ferric hematoxylin according to Weigert for 10 minutes, incubated with picric acid for 4 minutes, wash quickly with distilled water, stained with Ponceau B solution for 4 minutes, quickly wash with distilled water, incubate with phosphomolibdic acid for 10 minutes, stained with aniline blue according to Masson for 5 minutes, quickly wash with distilled water, dehydrate, cleared, assemble with Entellan and overlaid with coverslips.
Image Capturing and Analyses
The CD68 (+), CD163 (+) and CD11c (+) macrophage and the HIF1-α positive nuclei counts were performed manually by observing the whole area of the EAT sections using an Olympus BX51 photomicroscope equipped with a KY-F58 CCD camera (JVC) at 400x magnification. The total area of the observed section was then calculated using ImageJ software 1.49o version on the acquired EAT images and the number of macrophages and the number of nuclei HIF1-α (+) was expressed as a number / mm2 by dividing the number for the total area expressed in mm2.
For Caveolin-1 and fibrosis evaluation, slides were observed and images analyzed and stored using EVOS FL Auto Cell Imaging System with EVOS Onstage Incubator (Thermo Fisher Scientific) photomicroscope. The quantification of caveolin-1 was achieved by calculation of the optical density (OD). OD assay was performed on the whole section of the EAT by observing images at 200x magnification, using Color Threshold of ImageJ software 1.49o version. Image J software 1.49o version was also used to measure collagen deposition by calculating the percentage of blue staining, indicative of fibrosis, on total section area of EAT at 200x magnification by automated color deconvolution [20] and manual threshold correction where necessary. The percentage of fibrosis was adjusted by adipocyte size to eliminate the effects of different adipocyte sizes in measure fields [21]. The scoring was performed by one investigator who was blind to the identity of the samples.
Statistical Analysis
All results are shown as mean ± standard deviation (SD). Logarithmic transformation was performed for not normally distributed variables (HOMA index, adiponectin, insulin, glycemia, fibrosis, number of adipocytes nuclei HIF1-α (+), CD68 (+), CD163 (+) and CD11c (+) macrophages). Comparisons between CAD and non-CAD were performed using Student’s 2-tailed unpaired test. Simple Pearson correlations were used to test association between variables. A significance level of 5% was always adopted. All analyses were performed using SPSS (software version 17.0, SPSS, Chicago, IL, USA) and R program [22] (R project).
Results
Table 1 shows mean values ± standard deviation of anthropometric variables, serum adipokines, glycemia, insulin, HOMA index, CAV-1 in patients with and without CAD. Serum adiponectin was significantly lower in patients with CAD than in those without (8.25 ± 7.52 vs 15.06 ± 11.11, p<0.05), Figure 1 shows images and histograms of total CD68 (+), M2 anti-inflammatory CD163 (+), M1 pro-inflammatory CD11c macrophages and relative histograms in the EAT of patients with and without CAD. The number of total CD68 (74.25 ± 49.39 vs 37.33 ± 18.28, p<0.05), CD163 (57.75 ± 38.23 vs 31.11 ± 18.54, p<0.05) and CD11c (6.67 ± 4.73 vs 2.05 ± 1.13, p<0.01) were significantly higher in patients with CAD than in those without. It was interesting to note that in EAT of CAD patients we found pro-inflammatory CD11c (+) macrophages aggregated in crown like structure (CLS); CLS contained from a minimum of 6 to a maximum of 13 macrophages and surrounded what appears to be a single adipocyte. In total, 4 CLS were counted in 3 CAD patients (25% of the total CAD patients analyzed) while CLS were not observed in EAT of non-CAD patients. Furthermore, in EAT of both CAD and non CAD patients, CD163 (+) anti-inflammatory macrophages appeared dispersed throughout the adipose tissue, not organized in CLS.
Figure 2 show images and histograms respectively of fibrosis, nuclear HIF1-α and CAV-1 in the EAT of patients with and without CAD. Fibrosis was significantly higher in patients with CAD than in those without (3.86 ± 1.85 vs 1.45 ± 0.84, p<0.01), as well the number of nuclei HIF1-α (2.39 ± 1.64 vs 1.01 ± 0.94, p<0.05), while CAV-1 was significantly lower (0.0790 ± 0.0268 vs 0.0987 ± 0.0164, p<0.05). Table 2 shows correlations between fibrosis, nuclear HIF1-α and CAV-1 with anthropometric, metabolic variables and macrophages infiltration in the whole study sample. Fibrosis correlated with HIF1-α ( r= 0.644, p < 0.01) and M1 proinflammatory CD11c (+) macrophages (r=0.601, p<0.01); HIF1-α with total CD68 (+) (r= 0.5313, p<0.05), anti-inflammatory CD163 (+) macrophages (r= 0.673, p<0.001), pro-inflammatory CD11c (+) (r= 0.6385, p<0.01) whilst Caveolin-1 positively correlated with serum adiponectin (r= 0.4347, p<0.05).
Figure 1: a) Representative images of immunohistochemical staining and b) histograms of total CD68(+), M2 CD163(+) anti inflammatory and M1 CD11c (+) proinflammatory macrophages in the EAT of patients with coronary artery disease (CAD) compared to patients without CAD (NON CAD); in particular, aggregation of proinflammatory M1 CD11c(+) macrophages in CLS is shown in EAT of CAD patients (images of total and anti-inflammatory macrophages: scale bar = 25μm, images of proinflammatory macrophages: scale bar = 10μm. histograms = mean ± SD).
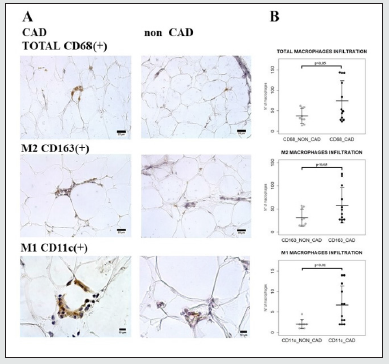
Figure 2: a) Representative images and b) histograms of fibrosis, number of adipocytes nuclei HIF1-α (+) and CAV-1 OD in the EAT of patients with coronary artery disease (CAD) compared to patients without CAD (non-CAD) (scale bar = 25μm images of fibrosis and HIF1-α, scale bar=50μm images of CAV-1; histograms = mean ± SD).
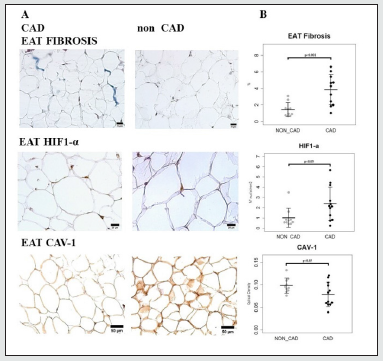
Table 1: Study Sample Characteristics.
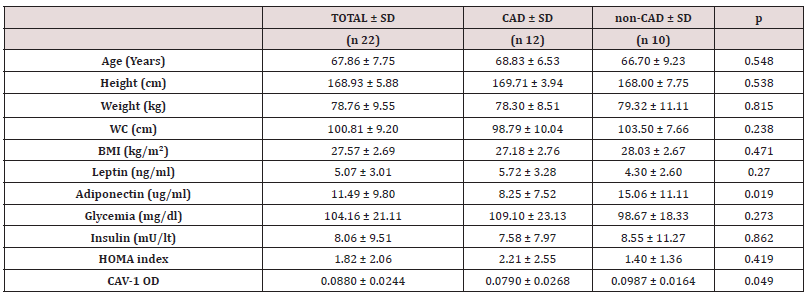
Abbreviations: CAD, Patients with Coronary Artery Disease; non-CAD, Patients without Coronary Artery Disease; SD, Standard Deviation; WC, Waist Circumference; BMI, Body Mass Index; HOMA index, Homeostasis Model Assessment; M CD68 (+), Total Macrophages; M CD163 (+), Anti-inflammatory Macrophages; M CD11c (+), Proinflammatory Macrophages; HIF1-α, Hypoxia-inducible factor 1-alpha
Table 2: Sample Pearson Correlations between CAV-1 OD (CAV-1), number of adipocytes nuclei HIF1-α (+) (HIF1-α) and fibrosis with main variables of the study.
Discussion
Our study shows that CAD patients display greater EAT inflammation, fibrosis and HIF1-a and lower CAV-1 than those without as well that fibrosis, macrophages infiltration and HIF1α are strongly interrelated each other. Moreover our study shows a significant association between CAV-1 and serum adiponectin. EAT thicknesses has been shown to be related with severity and progression of CAD, both in obese and non obese people [23]. Quality, besides quantity, of EAT has been shown to be involved in heart diseases, in particular CAD [24,25]. EAT macrophages infiltration and fibrosis have raised great interest in the last year and considered landmark of its dysfunction [23,26]. We observed greater pro-inflammatory macrophages infiltration in patients with CAD than in those without in agreement with previous observations of [27], who found a shift of macrophages toward a proinflammatory M1 state in EAT of CAD patients [27] and with those of [8]. who found a greater ratio of pro-inflammatory macrophages to total macrophages in CAD patients [8].
Interestingly we found that pro-inflammatory CD11c (+) macrophages were aggregated in CLS in the EAT of CAD patients but not in the EAT of those without. A CLS macrophages aggregation localized around dead adipocytes, where they fuse to form syncytia that sequester and scavenge the residual “free” adipocyte lipid droplet, was first detected by Cinti el al in AT of obese mice and humans [28], than observed by Patel et al, in the EAT of animal model ACE2 knockout high-fat diet feed [29] and by Pierzynová et al. in the EAT of CAD patients [30].
In addition to greater inflammation, we also observed greater fibrosis in the EAT of CAD patients than in those without. Fibrosis is the result of an excessive accumulation of extracellular matrix components such as collagen and fibronectin in damaged tissue, associated with organ dysfunction and death [31,32]. Jiang et al. showed a large number of collagen fibers deposition and also a significant increase in the expression of different types of collagen proteins such as collagen type III alpha 1 chain (Col3A1), collagen type I alpha 1 chain (Col1A1), collagen type VI alpha 1, 2 and 3 chain (Col6A1, Col6A2, Col6A3) and collagen type XII alpha 1 chain (Col12A1) in the EAT of ischemic cardiomyopathy patients compared with the EAT of normal heart of donors who died in accidents [33]. Association between inflammation and fibrosis is a common feature of visceral adipose tissue in obese and diabetic subjects [34, 35, 36] and has been previously described even in the EAT [37,25].
As expected, HIF1-a was significantly higher in the EAT of our patients with CAD than in that of patients without. A significant association between Hypoxia and fibrosis was observed in our study sample. A link between HIF1-a and fibrosis in AT has been previously found [38] using a model of transgenic mice overexpressing HIF1-α in AT, showed that HIF1-α promotes fibrosis characterized by an increase in both I and III collagen types deposition in the extracellular tissue matrix [38]. Interrelationship between Hypoxia, inflammation and fibrosis has been observed in AT also in obese insulin resistant patients [39].
Both in vitro and in vivo hypoxia is involved in the pathogenesis of fibrosis by activating the transcription factor HIF1-α [11], that promotes fibrogenesis through hydroxylation and up-regulation of collagen secretion, by activating the transforming growth factor β pathway, facilitating epithelial to mesenchymal transition and inducing connective tissue growth factor in fibroblast [11]. HIF1-α may also promote AT inflammation through fibrosis and macrophage recruitment leads to adipocyte cell death that causes further macrophage recruitment and inflammation [40] and or by induction of macrophages markers such as CD68, F4/80, by induction of monocyte chemo-attractants factors [38], which induce chemotaxis of both M1 and M2 polarized macrophages culture [41,42]. Studies in animal models, demonstrated that inhibition leads to amelioration of fibrosis and inflammation38 suggesting that HIF‐1α is a causal factor in producing inflammation and fibrosis [40].
We also observed lower Caveolin-1 in EAT of patients with CAD than in those without. Several studies conducted on caveolin-1 knockout mice have revealed that the absence of Caveolin-1 is associated with dilated cardiomyopathy, right ventricular dilation, concentric left ventricular hypertrophy and reduction in left ventricular systolic function [14, 43, 44], over expression of the pro-inflammatory cytokines compared to wilde type mice [46]. A reduction in the expression of Caveolin-1 has been shown in the AT of obese and aging patients [47], but to our best knowledge, a decline of CAV-1 in the EAT of CAD patients has not yet been described.
In our study, CAV-1 was associated with serum adiponectin. A link between CAV-1 and adiponectin was previously found [48], in transgenic mice over expressing adiponectin [48]. Adiponectin, an adipokine abundantly present in human plasma, is known to protect against the development of ischemic heart disease. Reduced serum levels of adiponectin are associated with CAD [49, 50]. Adiponectin protein in EAT was significantly lower in patients with CAD than in those without [51], adiponectin gene expression in EAT was found to be significantly lower in subjects with diabetes than in those without [52] as well as in patients with metabolic syndrome than in those without [53].
Studies have shown that adiponectin has anti-inflammatory effect by promoting IL-10 production, inhibiting tumor necrosis factor alpha production by macrophages [54], promoting antiapoptotic and anti-oxidative activities [55]. However, our results seem to suggest that adiponectin may decline CAD risk even by determining an increase of CAV-1. Some limitations of our study should be recognized. Firstly, as only men were included in the study sample, the results cannot be generalized and transferred to women; secondly the study design does not permit the evaluation of cause effect mechanisms, but just to make comparison and to identify association and the modest sample size may have mitigated against demonstrating other statistical association. Finally our study failed to show any association between obesity and fat distribution with indices of dysfunctional EAT probably due to the fact that patients with and without CAD were matched for BMI and waist circumference.
In conclusion, our study shows that increase in inflammation, fibrosis and hypoxia as well decline in Cav-1 characterized dysfunctional EAT in CAD. Our data seem also to suggest that hypoxia may account for both EAT inflammation and fibrosis increase. Finally, our results seem to suggest that adiponectin may decline CAD risk even by determining an increase of CAV-1.
Conflict of Interest:
None
Acknowledgments
We thank the staff of the research center LURM, as well as of the CPT (Technology Platform Center), for the precious support during the experimental research.
References
- Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk Kazberuk A, Malyszko J (2018) Patients with atrial fibrillation and coronary artery disease-Double trouble. Adv Med Sci 63(1): 30-35.
- Khera AV, Kathiresan S (2017) Genetics of coronary artery disease: Discovery, biology and clinical translation. Nat Rev Genet 18(6): 331-344.
- Nagy E, Jermendy AL, Merkely B, Maurovich Horvat P (2017) Clinical importance of epicardial adipose tissue. Arch Med Sci 13(4):864-874.
- Patel VB, Shah S, Verma S, Oudit GY (2017) Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail Rev 22(6): 889-902.
- Le Jemtel TH, Samson R, Ayinapudi K, Singh T, Oparil S (2019) Epicardial Adipose Tissue and Cardiovascular Disease. Curr Hypertens Rep 21(5): 36.
- Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, et al. (2011) Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol 10: 2.
- Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, et al. (2011) Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J 52(3): 139-142.
- Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, et al. (2011) Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 58(3): 248-255.
- Abe I, Teshima Y, Kondo H, Kaku H, Kira S, et al. (2018) Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 15(11): 1717-1727.
- Abe H, Semba H, Takeda N (2017) The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J Atheroscler Thromb 24(9): 884-894.
- Xiong A, Liu Y (2017) Targeting Hypoxia Inducible Factors-1α As a Novel Therapy in Fibrosis. Front Pharmacol 8: 326.
- O Rourke SA, Dunne A, Monaghan MG (2019) The Role of Macrophages in the Infarcted Myocardium: Orchestrators of ECM Remodeling. Front Cardiovasc Med 6: 101.
- Roth DM, Patel HH (2011) Role of caveolae in cardiac protection. Pediatr Cardiol 32(3): 329-333.
- Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, et al. (2002) Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A 99(17): 11375-11380.
- Zheng YZ, Boscher C, Inder KL, Fairbank M, Loo D, et al. (2011) Differential impact of caveolae and caveolin-1 scaffolds on the membrane raft proteome. Mol Cell Proteomics 10(10): 110.007146.
- Chettimada S, Yang J, Moon HG, Jin Y (2015) Caveolae, caveolin-1 and cavin-1: Emerging roles in pulmonary hypertension. World J Respirol 5(2):126-134.
- Haddad D, Al Madhoun A, Nizam R, Al-Mulla F (2020) Role of Caveolin-1 in Diabetes and Its Complications. Oxid Med Cell Longev 2020: 9761539.
- Parton RG, Tillu VA, Collins BM (2018) Caveolae. Curr Biol 28(8): 402-405.
- Fridolfsson HN, Patel HH (2013) Caveolin and caveolae in age associated cardiovascular disease. J Geriatr Cardiol 10(1): 66-74.
- Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, et al. (2011) Assessment of diffuse myocardial fibrosis in rats using small-animal Look-Locker inversion recovery T1 mapping. Circ Cardiovasc Imaging 4(6): 636-640.
- Liu Y, Aron Wisnewsky J, Marcelin G, Genser L, Le Naour G, et al. (2016) Accumulation and Changes in Composition of Collagens in Subcutaneous Adipose Tissue After Bariatric Surgery. J Clin Endocrinol Metab 101(1): 293-304.
- R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Packer M (2018) Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J Am Coll Cardiol 71(20): 2360-2372.
- Vianello E, Dozio E, Bandera F, Schmitz G, Nebuloni M, et al. (2019) Dysfunctional EAT thickness may promote maladaptive heart remodeling in CVD patients through the ST2-IL33 system, directly related to EPAC protein expression. Sci Rep 9(1): 10331.
- Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F, Carbone F (2019) Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol 278: 254-260.
- Iacobellis G (2015) Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11(6): 363-371.
- Vianello E, Dozio E, Arnaboldi F, Marazzi MG, Martinelli C, et al. (2016) Epicardial adipocyte hypertrophy: Association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr Metab Cardiovasc Dis 26(3): 246-253.
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, et al. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46(11): 2347-2355.
- Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, et al. (2016) ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 65(1): 85-95.
- Pierzynová A, Šrámek J, Cinkajzlová A, Kratochvílová H, Lindner J, et al. (2019) The number and phenotype of myocardial and adipose tissue CD68+ cells is associated with cardiovascular and metabolic disease in heart surgery patients. Nutr Metab Cardiovasc Dis 29(9): 946-955.
- Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028-1040.
- Zeisberg M, Kalluri R (2013) Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 304(3): 216-225.
- Jiang DS, Zeng HL, Li R, Huo B, Su YS, et al. (2017) Aberrant Epicardial Adipose Tissue Extracellular Matrix Remodeling in Patients with Severe Ischemic Cardiomyopathy: Insight from Comparative Quantitative Proteomics. Sci Rep 7: 43787.
- Catalán V, Gómez Ambrosi J, Rodríguez A, Ramírez B, Rotellar F, et al. (2011) Increased circulating and visceral adipose tissue expression levels of YKL-40 in obesity-associated type 2 diabetes are related to inflammation: Impact of conventional weight loss and gastric bypass. J Clin Endocrinol Metab 96(1): 200-209.
- Pasarica M, Gowronska Kozak B, Burk D, Remedios I, Hymel D, et al. (2009) Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 94(12): 5155-5162.
- Shang C, Sun W, Wang C, Wang X, Zhu H, et al. (2019) Comparative Proteomic Analysis of Visceral Adipose Tissue in Morbidly Obese and Normal Weight Chinese Women. Int J Endocrinol 2019:2302753.
- Packer M (2019) Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: The potential mediating influence of epicardial adipose tissue. Cardiovasc Diabetol 18(1): 121.
- Halberg N, Khan T, Trujillo ME, Wernstedt Asterholm I, Attie AD, Sherwani S, et al. (2009) Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29(16): 4467-4483.
- Lawler HM, Underkofler CM, Kern PA, Erickson C, Bredbeck B, et al. (2016) Adipose Tissue Hypoxia, Inflammation, and Fibrosis in Obese Insulin-Sensitive and Obese Insulin-Resistant Subjects. J Clin Endocrinol Metab 101(4): 1422-1428.
- Warbrick I, Rabkin SW (2019) Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes Rev 20: 701-712.
- Xuan W, Qu Q, Zheng B, Xiong S, Fan GH (2015) The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol 97(1): 61-69.
- Sierra Filardi E, Nieto C, Domínguez Soto A, Barroso R, Sánchez Mateos P, Puig Kroger A, et al. (2014) CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 192(8): 3858-3867.
- Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, et al. (2003) Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol 284(2): 457-474.
- Murata T, Lin MI, Huang Y, Yu J, Bauer PM, et al. (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204(10): 2373-2382.
- Codrici E, Albulescu L, Popescu ID, Mihai S, Enciu AM, et al. (2018) Caveolin-1-Knockout Mouse as a Model of Inflammatory Diseases. J Immunol Res 2018: 2498576.
- Razani B, Combs TP, Wang XB, Frank PG, Park DS, et al. (2002) Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem 277(10): 8635-8647.
- Dou H, Feher A, Davila AC, Romero MJ, Patel VS, et al. (2017) Role of Adipose Tissue Endothelial ADAM17 in Age-Related Coronary Microvascular Dysfunction. Arterioscler Thromb Vasc Biol 37(6): 1180-1193.
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, et al. (2004) A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145(1): 367-383.
- Shibata R, Ouchi N, Ohashi K, Murohara T (2017) The role of adipokines in cardiovascular disease. J Cardiol 70(4): 329-334.
- Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, et al. (2007) Serum adiponectin is a predictor of coronary heart disease: A population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab 92(2): 571-576.
- Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, et al. (2005) Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 29(6): 251-255.
- Bambace C, Sepe A, Zoico E, Telesca M, Olioso D, et al. (2014) Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels 29(1): 42-48.
- Teijeira Fernandez E, Eiras S, Shamagian LG, Somoza AS, Delgado C, et al. (2011) Lower epicardial adipose tissue adiponectin in patients with metabolic syndrome. Cytokine 54(2): 185-190.
- Robinson K, Prins J, Venkatesh B (2011) Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care 15(2): 221.
- Hui X, Lam KS, Vanhoutte PM, Xu A (2012) Adiponectin and cardiovascular health: An update. Br J Pharmacol 165(3): 574-590.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




