Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Mini Review(ISSN: 2638-5910) 
Beyond glucose control agents: Sodium-Glucose Co-transporter 2 Inhibitors in Heart failure Volume 3 - Issue 4
Nikolaos Karamichalakis*
- Department of Cardiology, Hygeia Hospital, Greece
Received:May 31, 2021; Published: June 10, 2021
Corresponding author: Nikolaos Karamichalakis, Department of Cardiology, Hygeia Hospital, Athens, Greece
DOI: 10.32474/ADO.2021.03.000167
Introduction
HF and T2D often coexist and the maleficent effects of this coupling prompted the US Food and Drug Administration to set hypoglycaemic drugs clinical trials, designed to rule out cardiovascular harm and promote cardioprotection in the year 2008 [1]. In the line of this strategic motivation, cardiovascular outcome trials (CVOTs) with a novel class of hypoglycaemic drugs, the sodium-glucose co-transporter 2 (SGLT2) inhibitors, emerged and surprisingly demonstrated positive cardiovascular outcomes, mainly due to a decline in heart failure (HF) risk. The EMPAREG OUTCOME trial with empagliflozin, the CANVAS Program with canagliflozin, the DECLARE-TIMI 58 with dapagliflozin and the most recent VERTIS-CV with ertugliflozin, are the focal point and impel novel heart failure therapy strategies, as no other known CVOT has displayed such benefit on heart failure events so far [2-4]. The so far proposed cardioprotective mechanisms of SGLT2 inhibition include diuresis and natriuresis, decline in arterial blood pressure, erythropoiesis, enhanced heart energy metabolism, decline in inflammation, inhibition of the sympathetic nervous system, reduce in oxidative stress and improved endothelium function, among others [5-8]. In this review, we present a comprehensive, evidence-based thesis about the treatment with SGLT2 inhibitors (SGLT2i) in cardiovascular patients with or without T2D, their effect in HF and analyse the major published and ongoing SGLT2i trials. We also shed light upon the SGLT2i mechanisms of action and provide practical considerations about their application in HF patients, based on current recommendations.
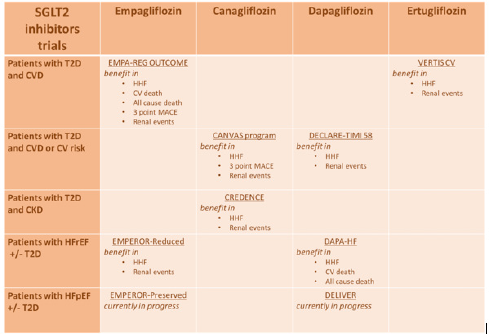
SGLT2 Inhibitors and Heart Failure: The Clinical Trials
SGLT2i Cardiovascular Outcome Trials in T2D Patients
The EMPA-REG OUTCOME Trial
Empagliflozin is the first SGLT2i and glucose-lowering medication to receive approval for cardiovascular protection. In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, patients with T2D and known cardiovascular disease (CVD) who received empagliflozin presented reduced risk of 3 point MACE (a composite of death from CV causes, non-fatal MI or non-fatal stroke), as well as significant reduction in all-cause mortality, cardiovascular mortality and HF hospitalization (35% reduction compared with placebo, HR 0.65; 95% CI [0.50–0.85]; p=0.002).(2) Post hoc analysis of the trial demonstrated that the risk reduction of all cause/cardiovascular mortality and HF hospitalizations with empagliflozin was obvious in patients with and without HF, although patients with HF had higher rates of the aforementioned outcomes [9]. In addition, empagliflozin had a beneficial impact in HF route by lowering not only sudden cardiac death but also death due to pump failure events [10]. The HF patient in the EMPA-REG OUTCOME trial was defined as a patient i) with known HF from the beginning, ii) or had been hospitalised for HF or iii) or presented HF during the course of the trial.
The CANVAS Program
The Canagliflozin Cardiovascular Assessment Study (CANVAS) program, which included the CANVAS and CANVAS-R (renal) studies, assessed the use of canagliflozin in patients with T2D and i) known CVD at baseline or ii) at least two risk factors for CVD [11]. In consistency with the EMPA-REG OUTCOME trial, the CANVAS trial showed substantial improvements in HF hospitalisation rate for HF for canagliflozin versus placebo (33% reduction compared with placebo, HR 0.67; 95% CI [0.52–0.91]) [11]. The results for the CANVAS program were driven by a reduction in HF hospitalizations, but with no significant reduction in CV death was noticed.
DECLARE-TIMI 58 Trial
The Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI 58) trial compared dapagliflozin vs. placebo in T2D patients with either atherosclerotic CVD at baseline or multiple risk factors [4]. This trial demonstrated a significant reduction in its co-primary endpoint which was HF hospitalisation for HF and CV death (HR 0.83; 95% CI [0.73–0.95]; p=0.005), mostly due to a lower rate in HF hospitalisation (27% reduction compared with placebo, HR 0.73; 95% CI [0.61–0.88]). Similar results were shown in dapagliflozin treatment for patients with and without HF at baseline [4]. A nonstatistically significant reduction in the second primary endpoint which included CV death, MI or stroke was noticed, possibly because of the lower overall rate of CV events in this trial compared with other SGLT2i CVOTs [2,4,11].
The VERTIS CV trial
In the most recent Evaluation of Ertugliflozin efficacy and Safety Cardiovascular outcomes trial (VERTIS-CV), the fourth to be released SGLT2i was compared to placebo in T2D patients with CVD [12]. After a follow-up of 3.5 years, 5499 out of 8246 randomised patients who received ertugliflozin did not present increase in terms of MACE defined as death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke [12]. In contrast to the other SGLT2i CVOTs, in the VERTIS CV trial, the rate of CV death or HF hospitalization (the first key secondary composite outcome) did not differ significantly between the compared groups. While the participants characteristics in the trial were similar to the other SGLT2i CVOTs, there is not an obvious explanation for the noninferiority conducted by the statistical analysis [12]. Advances in terms of secondary prevention medication in the recent years following the previous CVOTs may had an effect on VERTIS CV. Another possible reason is a variation in the SGLT2 class effect, as ertugliflozin presents higher selectivity to SGLT2 compared to SGLT1 [13].
Metanalyses and Real-World Data
In the so far published metanalyses, SGLT2i have demonstrated a broad class effect in reducing HF hospitalisations in patients with or without baseline CVD and but also in preserving renal function [14,15]. Despite the encouraging results of the aforementioned trials, a semantic disparity is noticed in rates of CV death. Excluding patients with risk factors, in patients with established CVD, empagliflozin was the only agent in class that reduced significantly all-cause and CV mortality [9,15]. In addition, the EMPAREGOUTCOME had a more lenient inclusion protocol in terms of renal function, allowing patients with lower estimated glomerular filtration rate (eGFR) to participate. Patients with eGFR < 60 mL/ min had a notable portion of 25.9% in EMPA-REG, while that percentage was 20.1% and 7.4% in the CANVAS and DECLARE-TIMI 58 trial accordingly [4, 9, 11, 16].
Metanalyses have shown that the SGLT2i class effect of risk reduction in terms of HF hospitalisation is consistent among a wide spectrum of renal function and it is profound in patients with greater renal dysfunction [15]. The most recent Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) trial included patients with T2D and nephropathy described as an eGFR of 30 to < 90 mL/min and albuminuria [17]. At baseline, over 50% of the participants had established CVD and 14.8% suffered from HF. During a median follow-up of 2.62 years, this trial demonstrated a considerable reduction in terms of a composite of HF hospitalisation and CV death (HR 0.69; 95% CI [0.57–0.83]; p<0.001) and HF hospitalisation (HR 0.61; 95% CI [0.47–0.80]; p<0.001) for patients who received canagliflozin instead of placebo [17].
In real world setting, CV outcomes and HF reduction risk associated with the use of SGLT2i have been evaluated in the CVDREAL and EMPRISE studies.(18-20) The CVD-REAL study had access in six countries registries and compared initiation of SGLT2i vs other glucose-lowering drugs, in 309,056 T2D patients with or without CVD. SGLT2i demonstrated beneficial effect by reducing HF hospitalisations (HR 0.61, 95% CI 0.51–0.73, p < 0.001); all-cause mortality (HR 0.49, 95% CI 0.41–0.57, p < 0.001), and a composite of HF hospitalisation or all-cause death (HR 0.54, 95% CI 0.48–0.60, p < 0.001) [18, 20].
The EMPRISE study is currently in progress and evaluates the safety and efficacy of newly started empagliflozin or sitagliptin in 232,000 US T2D patients in standard care setting [19]. About 32,000 paired patients, with or without CVD, who received empagliflozin have shown a 50% rick reduction in HF hospitalisations (HR 0.50, 95% CI 0.28–0.91, p = NA), after 5 months of follow-up [16,19]. The fourth SGLT2i, ertugliflozin, is expected soon to be released and results of its CVOT metanalyses and real-world data are underway [12, 21].
SGLT2i Trials in HFrEF Patients with or without T2D DAPA-HF is the first SGLT2i CVOT to evaluate Dapagliflozin as HF treatment in HFrEF patients with or without T2D [22]. Dapagliflozin in DAPA-HF was compared to placebo in a multicentre Phase III trial participating 4,744 patients with NYHA class II, III or IV HF and LVEF equal or less than 40%. Inclusion criteria consisted of i) NT-proBNP equal or more than 600 pg/ml or ii) 400 pg/ml if they had a HF hospitalisation within a year or iii) 900 pg/ml if they suffered from atrial fibrillation or atrial flutter on inclusion ECG. Patients randomised were on optimal HF guideline-oriented medication, including an ACEi, an ARB, or sacubitril–valsartan plus a beta-blocker, unless contraindicated and received dapagliflozin 10 mg once daily or placebo. There were no substantial differences in initial therapies between the two groups∙ 45% of the participants suffered from T2D and 55% did not [22].
Dapagliflozin 10 mg od demonstrated a significant reduction in HF deterioration (aka HF hospitalisation or urgent HF visit) and CV death (HR 0.74, 95% CI 0.65–0.85, p = 0.001), both in T2D patients (HR 0.75, 95% CI 0.63–0.90, p = NA) and T2D-free patients (HR 0.73, 95% CI 0.60–0.88, p=NA) [22]. Primary and recurrent HF hospitalisations were significantly lower for the ones receiving dapagliflozin compared to placebo [23]. In DAPA HF, when on dapagliflozin, the number of patients needed to treat (NNT) in order to prevent a primary event was 21 (95% CI 15– 38). A non-significant 29% reduction in terms of worsening renal function, was also noticed in the dapagliflozin group (HR 0.71; 95% CI [0.44–1.16]). A post hoc analysis revealed that patients receiving sacubitril/valsartan at baseline, despite representing a mere 10% of the trial participants, had the same added benefit of SGLT2i therapy and the beneficial effect is consistent regardless of background HF therapy [24].
In The Empagliflozin Compared With Placebo On Exercise Ability and Heart Failure Symptoms in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPERIAL-Reduced) study 312 HFrEF patients with or without T2D were evaluated in terms of exercise capacity via a 6 minute walk test and patient-reported HF outcomes [25]. Empagliflozin was well tolerated and approved for safety but results for the primary outcome were neutral [25].
In the EMPEROR-Reduced Phase III trial, 3,730 patients with HF NYHA class II, III or IV and LVEF equal or less than 40%, received either empagliflozin 10 mg od or placebo [26]. Like DAPA HF, patients randomised were already on optimal HF guidelineoriented medication. Inclusion NT-proBNP level criteria were in concordance with LVEF at initation of the trial: ≥600 pg/ml for patients with LVEF ≤30%, ≥1,000 pg/ml for LVEF 31–35%, ≥2,500 pg/ml for LVEF 36–<40% and when in AF the NT-proBNP inclusion threshold was doubled. Patients with eGFR less than 20 ml/min/1.73m2 or in dialysis were excluded from the trial [26]. Again, like DAPA HF, there were no substantial differences in initial therapies between the two groups· 49.5% of the participants suffered from T2D.
After a median follow-up of 16 months, the primary outcome (combined risk of CV death and HF hospitalisation) was addressed in 361 of 1,863 patients (19.4%) in the empagliflozin group and in 462 of 1,867 patients (24.7%) in the placebo group (HR 0.75; 95% CI [0.65–0.86]; p<0.001), mainly because of reduced rates of HF hospitalisation in the empagliflozin arm (HR 0.70; 95% CI [0.58–0.85]; p<0.001) [27]. Empagliflozin-treated patients had a lower risk of serious renal events and demonstrated a significant reduction in the rate of renal disease progression, as measured by eGFR slope over time (–0.55 versus –2.28 ml/min/1.73m2 per year; absolute difference 1.73 ml/min/1.73m2 per year; 95% CI [1.10– 2.37]; p<0.001).(27) On the other hand, empagliflozin did not affect CV and all-cause death rates (HR 0.92; 95% CI [0.75–1.12] and HR 0.92; 95% CI [0.77–1.10] accordingly) [2, 26, 27]. The beneficial effect of empagliflozin was observed throughout the range of renal function, regardless of RAAS inhibition and ARNI administration, in patients with or without T2D [27].
SGLT2i Trials in HFpEF Patients
The first SGLT2i trial dedicated in HFpEF patients, but restricted in 312 participants, was the Empagliflozin Compared With Placebo on Exercise Ability and Heart Failure Symptoms, In Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPERIAL-Preserved) trial [25]. It evaluated the effect of empagliflozin administration on exercise capacity but after 12 weeks no significant difference was shown between the empagliflozin and placebo groups in terms of 6-minute walk test or KCCQ total symptom scores [25].
Among the heterogeneous groups of HF patients with preserved ejection fraction, two major SGLT2i CVOTs are underway. In the soon to be completed Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPERORPreserved; NCT03057951) trial, 5,988 HFpEF patients with or without T2D will be randomised to receive empagliflozin or placebo [28]. In the same route, the Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER; NCT03619213) trial will evaluate administration of dapagliflozin or placebo as add-on to standard therapy in 6,100 HFpEF patients with or without T2D [29].
Dapagliflozin Effect on Exercise Capacity Using a 6-minute Walk Test in Patients With Heart Failure With Preserved Ejection Fraction (DETERMINE-Preserved; NCT03877224) is a another randomised controlled trial to evaluate the effect of dapagliflozin on exercise capacity and QoL in 504 HFpEF patients. The SOLOIST trial with the SGLT1/SGLT2 inhibitor sotagliflozin, which was early terminated due to financial issues, included both HFpEF and HFrEF patients and could add valuable information provided sufficient data were collected [23]. The HFpEF phenotype, albeit common in real world setting, is not often represent in trials and hard to evaluate. A CANVAS program derived, post-hoc analysis of a HFpEF subgroup demonstrated reduction in rates of hospitalisation and death events (HR 0.83; 95% CI [0.55–1.25]), but because of the limited HFpEF sample it could not reach statistical significance [30]. Likewise, a DECLARE_TIMI 58 study derived, post-hoc analysis of a HFpEF subgroup demonstrated reduction in HF hospitalisations (HR 0.74; 95% CI [0.48–1.14]) but because only 4.7% of the participants were HFpEF patients, these results should be taken with a grain of salt [31].
SGLT2i Mechanisms of Cardiovascular Protection
Despite the intriguing trial results, the SGLT2i mechanisms of CV protection are not totally understood and some parts remain covered in mystery. SGLT2i inhibitors have demonstrated a broad effect in the cardio–renal axis, affecting many systems, regardless of glycaemic control. The SGLT2 channel, located in the proximal kidney tubule, is responsible for the majority of glucose reabsorption [6,7]. SGLT2i promote glycosuria and natriuresis by restraining glucose and Na+ reabsorption. Through improvement in blood glucose and HbA1c levels, arterial blood pressure lowering and body weight loss, they seem to protect against CVD and HF [6,32]. Along with glycosuria and natriuresis, SGLT2i inhibitors push for uricosuria [33,34]. Although impressive, these effects of SGLT2i do not explain thoroughly the outcomes observed in CVOTs compared to placebo, shifting the SGLT2i beneficial impact beyond glycaemic or blood pressure control.
SGLT2 inhibitors retain intravascular volume and reduce interstitial volume, in contradistinction to diuretics. By reducing preload and afterload, they boost improvement in endothelial function, arterial elasticity and an overall upturn in hemodynamic parameters [6]. Incoming data imply that SGLT2i prevent electrolyte disturbances, neurohormonal activation and renal function deterioration caused commonly by diuretics [35, 36]. Along with volume contraction, SGLT2i promote haemoconcentration, probably due to intrinsic renal processes, such as increased erythropoietin (EPO) production and restoration of the tubulointerstitial oxygenation [5]. The failing myocardium in HF patients suffers from underutilisation of glucose and fatty acids, thus creating an accumulation of metabolic products. SGLT2i increase the oxidation of ketone bodies, by enhanced lipid mobilization caused by glycosuria, lowered plasma glucose and insulin levels, along with an upraise in plasma glucagon [37]. This so called “thrifty substrate” hypothesis, suggests that SGLT2i improve the heart’s energetic and metabolic function by producing and consuming ketone bodies who are more energy-efficient than fatty acids [38].
Another proposed mechanism of SLGT2i related cardio-renal protection, implies inhibition of the Na+/H+ exchangers in the heart and kidney [6,39]. Cardiac and renal Na+/H+ exchange inhibition reduces cytoplasmic Na+ and Ca2+, which in cardiomyocytes, translates to an upturn in contractility and mitochondrial function [6, 40]. Preliminary in-vitro studies have also described the positive effect of SGLT2i on cytokine production, adipokine expression and epicardial adipose tissue mass [6,40]. SGLT2i reduce the maximum renal transport capacity for glucose reabsorption leading to a lower cut-off for glycosuria (60–90 g/day). Because of the insulinindependent mechanism to lower blood glucose, patients receiving SGLT2is are at a limited risk of hypoglycemia [41,42]. Thus, in the three large prospective CVOTs, hypoglycemia was at similar rates in the SGLT2i and in the placebo groups [2, 4, 11]. Several studies with Empagliflozin or Dapagliflozin are now conducted, investigating the effect of SGLT2i on cardiac metabolic and energetic function and their results will lead us to a better comprehension of the SGLT2i actions and mechanism of cardioprotection [43-45].
SGLT2i in Clinical Practice
After the phenomenal results of the DAPA-HF and EMPERORReduced trials, SGLT2i are now candidates for joining the big three (ACEIs/ARBs, MRAs and beta blockers) as disease modifying agents in HFrEF patients. Empagliflozin was the first of its class to enter the 2016 ESC HF guidelines: “Empagliflozin should be considered in patients with T2D in order to prevent or delay the onset of HF” with a IIa class of recommendation and level of evidence B [46]. After 3 years, in the 2019 ESC guidelines for diabetes and CV protection all three SGLT2i (canagliflozin, dapagliflozin or empagliflozin) were included and were recommended for T2D patients with established CVD or at high/very high rick for CVD with an Ia class of recommendation [47]. Canagliflozin, dapagliflozin and empagliflozin were recommended for T2D patients to lower the risk of HF hospitalisation, again with an Ia class of recommendation [47].
In a 2019 position paper, joined by ESC/HFA, empagliflozin canagliflozin and dapagliflozin gained approval as recommended therapies in T2D patients and high CV risk for the prevention of HF hospitalisations, after demonstrating a substantial reduction in the risk of hospitalisation for HF across the spectrum of CV risk and regardless of a history of HF [48]. Also, in a most recent update by ESC/HFA, SLT2i ertugliflozin gained the class recommendation for preventing HF hospitalization in T2D patients and established CV disease or at high CV risk [49]. The DAPA HF and EMPEROR REDUCED positive results on CV and all-cause death, along with the significant reduction noticed in HF hospitalisations, elevated dagliflozin and empagliflozin as the preferred SGLT2i in symptomatic HFrEF patients and administration should be pursued regardless of the presence of T2DM [49] (Table 1).
SGLT2: sodium-glucose co-transporter 2, T2D: type 2 diabetes, CVD: cardiovascular disease, CV: cardiovascular, CKD: chronic kidney disease, HFrEF: heart failure with reduced ejection fraction, HFpEF: heart failure with preserved ejection fraction.
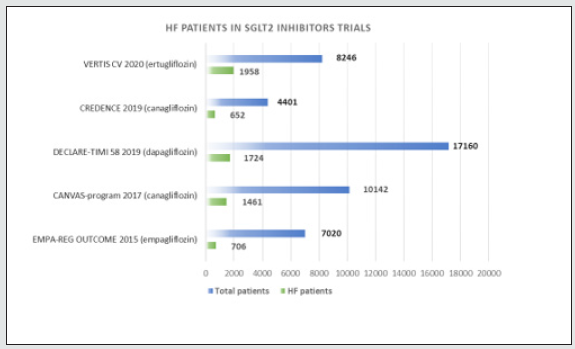
SGLT2i are associated with a minimal risk of drug-drug interactions and can be combined with all other glucose control agents [50]. On the other hand, due to their unique pharmacological profile and possible adverse effects, an individual benefit-risk evaluation must be taken before initiation [51]. Adverse events in patients receiving SGLT2i, such as urinary and genital infections, are not to be neglected, but can be correlated with predisposing conditions, such as volume depletion and euglycemic diabetic ketoacidosis [52]. Frail elderly patients who are prone to dehydration and orthostatic hypotension should be treated with caution. Other side effects, such as bone fractures and lower limb amputations are unexpected and remain poorly understood [53, 54]. Still, results regarding the aforementioned side effects are highly heterogeneous and real-world data seem to be reassuring [55] (Figure 1 and 2).
Figure 1: Class effect of Sodium-Glucose Co-transporter 2 inhibitors on cardiorenal outcomes. H HF: hospitalization for heart failure, CV: cardiovascular, MACE: major adverse cardiovascular events.
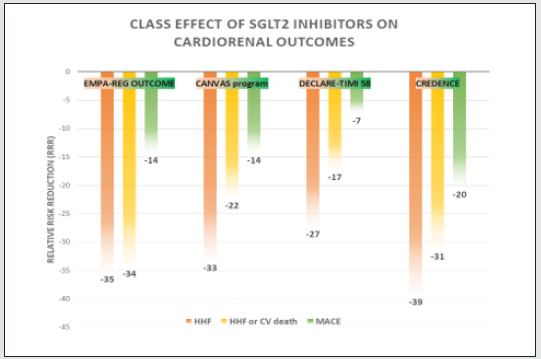
Figure 2: Participation of heart failure patients in Sodium-Glucose Co-transporter 2 inhibitors in cardiovascular outcome trials. HF: heart failure, SGLT2: Sodium-Glucose Co-transporter 2.
Conclusion
Despite advances in drug and device-therapy n HF patients, there is still an unmet need for disease‐modifying agents. SGLT2i have shown to offer cardiac and renal protection for HF (mostly HFrEF) patients, but also to reduce the risk for HF occurrence, regardless of glycaemic control, in patients with or without T2D. Addition of dapagliflozin and empagliflozin in the conventional recommended ACEi/ARB, MRA and beta-blocker algorithm is beneficial at any point during HFrEF course. The SGLT2i underlying mechanisms of action, although still under investigation, provide cardiac and renal benefit in many ways, complementary or collectively. Along with pending trials’ results, further trials are required to fully understand the SGLT2i pharmacokinetics, but also to determine whether these results can be applied in HFpEF patients, where no other known therapy has shown improved outcomes.
References
- American Diabetes Association (2020) 10 Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2020. Diabetes care 43(Suppl 1): 111-134.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine 373(22): 2117-2128.
- Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, et al. (2018) Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 137(4): 323-334.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. (2019) Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380(4): 347-357.
- Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV (2018) Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 20(3): 479-487.
- Lopaschuk GD, Verma S (2020 ) Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic to translational science 5(6): 632-644.
- Lam CSP, Chandramouli C, Ahooja V, Verma S (2019) SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. Journal of the American Heart Association 8(20): 013389.
- Verma S, Jüni P, Mazer CD (2019) Pump, pipes, and filter: Do SGLT2 inhibitors cover it all? Lancet 393(10166): 3-5.
- Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, et al. (2018) Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® European heart journal 39(5): 363-370.
- Packer M (2019) Reconceptualization of the Molecular Mechanism by Which Sodium-Glucose Cotransporter 2 Inhibitors Reduce the Risk of Heart Failure Events. Circulation 140(6): 443-445.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, et al. (2017) Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine 377(7): 644-657.
- Cannon CP, Pratley R, Dagogo Jack S, Mancuso J, Huyck S, et al. (2020) Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. The New England journal of medicine 383(15): 1425-1435.
- Cinti F, Moffa S, Impronta F, Cefalo CM, Sun VA, et al. (2017) Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: Evidence to date. Drug design, development and therapy 11: 2905-2919.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, et al. (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393(10166): 31-39.
- Monami M, Dicembrini I, Mannucci E (2017) Effects of SGLT-2 inhibitors on mortality and cardiovascular events: A comprehensive meta-analysis of randomized controlled trials. Acta diabetologica 54(1): 19-36.
- Brito D, Bettencourt P, Carvalho D, Ferreira J, Fontes Carvalho R, et al. (2020) Sodium-Glucose Co-transporter 2 Inhibitors in the Failing Heart: A Growing Potential. Cardiovascular drugs and therapy 34(3) :419-436.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, et al. (2019) Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. The New England journal of medicine 380(24): 2295-2306.
- Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, et al. (2019) Dapagliflozin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus and Previous Myocardial Infarction. Circulation 139(22): 2516-2527.
- Patorno E, Najafzadeh M, Pawar A, Franklin JM, Déruaz Luyet A (2020) The EMP agliflozin compaRative effectIveness and SafEty (EMPRISE) study programme: Design and exposure accrual for an evaluation of empagliflozin in routine clinical care. Endocrinol Diabetes Metab 3(1): 00103.
- Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, et al. (2017) Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 136(3): 249-259.
- Cannon CP, McGuire DK, Pratley R, Dagogo Jack S, Mancuso J, et al. (2018) Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). American heart journal 206: 11-23.
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. (2019) Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine 381(21): 1995-2008.
- Rosano G, Quek D, Martínez F (2020) Sodium-Glucose Co-transporter 2 Inhibitors in Heart Failure: Recent Data and Implications for Practice. Cardiac failure review 6: 31.
- Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, et al. (2020) Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. European heart journal 41(25): 2379-2392.
- Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, et al. (2020) Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. European heart journal 42(6): 700-710.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, et al. (2020) Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. The New England journal of medicine 383(15):1413-1424.
- Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, et al. (2019) Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: Rationale for and design of the EMPEROR-Preserved Trial. European journal of heart failure 21(10): 1279-1287.
- Williams DM, Evans M (2020) Dapagliflozin for Heart Failure with Preserved Ejection Fraction: Will the DELIVER Study Deliver?. Diabetes therapy: Research, treatment and education of diabetes and related disorders 11(10): 2207-2219.
- Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, et al. (2019) Effects of Canagliflozin on Heart Failure Outcomes Associated with Preserved and Reduced Ejection Fraction in Type 2 Diabetes Mellitus. Circulation 139(22): 2591-2593.
- Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, et al. (2019) Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 139(22): 2528-2536.
- Verma S, McMurray JJV, Cherney DZI (2017) The Metabolodiuretic Promise of Sodium-Dependent Glucose Cotransporter 2 Inhibition: The Search for the Sweet Spot in Heart Failure. JAMA cardiology 2(9): 939-940.
- Zhao Y, Xu L, Tian D, Xia P, Zheng H, et al. (2018) Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism 20(2): 458-462.
- Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, et al. (2014) SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharmaceutics & drug disposition 35(7): 391-404.
- Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, et al. (2016) Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: A meta-analysis of randomised controlled trials. Diabetologia 59(12): 2546-2551.
- Yurista SR, Silljé HHW, van Goor H, Hillebrands JL, Heerspink HJL, et al. (2020) Effects of Sodium-Glucose Co-transporter 2 Inhibition with Empaglifozin on Renal Structure and Function in Non-diabetic Rats with Left Ventricular Dysfunction After Myocardial Infarction. Cardiovascular drugs and therapy 34(3): 311-321.
- Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, et al. (2018) Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic to translational science 3(5): 575-587.
- Packer M (2017) Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus with That of Heart Failure. Circulation 136(16): 1548-1559.
- Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, et al. (2018) Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC heart failure 5(4): 642-648.
- Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61(10): 2108-2117.
- DeFronzo RA, Norton L, Abdul Ghani M (2017) Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nature reviews Nephrology 13(1): 11-26.
- Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J (2013) Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes, obesity & metabolism 15(9): 853-862.
- Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, et al. (2019) Empagliflozin in heart failure patients with reduced ejection fraction: a randomized clinical trial (Empire HF). Trials 20(1): 374.
- Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, et al. (2021) Associations of Empagliflozin With Left Ventricular Volumes, Mass, and Function in Patients With Heart Failure and Reduced Ejection Fraction: A Substudy of the Empire HF Randomized Clinical Trial. JAMA cardiology.
- Åkerblom A, Oldgren J, Latva Rasku A, Johansson L, Lisovskaja V, et al. (2019) Effects of DAPA gliflozin on CARDiac substrate uptake, myocardial efficiency, and myocardial contractile work in type 2 diabetes patients-a description of the DAPACARD study. Upsala journal of medical sciences 124(1): 59-64.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal 37(27): 2129-2200.
- Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, et al. (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European heart journal 41(2): 255-323.
- Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, et al. (2020) European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose-lowering drugs in patients with heart failure. European journal of heart failure 22(2): 196-213.
- Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, et al. (2020) Heart Failure Association of the European Society of Cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. European journal of heart failure 22(11): 1984-1986.
- Davies MJ, D Alessio DA, Fradkin J, Kernan WN, Mathieu C (2018) Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12): 2669-2701.
- Lupsa BC, Inzucchi SE (2018) Use of SGLT2 inhibitors in type 2 diabetes: Weighing the risks and benefits. Diabetologia 61(10): 2118-2125.
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, et al. (2015) Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment with Sodium-Glucose Cotransporter 2 Inhibition. Diabetes care 38(9): 1687-1693.
- Scheen AJ (2018) Does lower limb amputation concern all SGLT2 inhibitors?. Nature reviews Endocrinology 14(6): 326-328.
- Taylor SI, Blau JE, Rother KI (2015) Possible adverse effects of SGLT2 inhibitors on bone. The lancet Diabetes & endocrinology. 3(1): 8-10.
- Toulis KA, Bilezikian JP, Thomas GN, Hanif W, Kotsa K, et al. (2018) Initiation of dapagliflozin and treatment-emergent fractures. Diabetes Obes Metab 20(4): 1070-1074.
- Yuan Z, DeFalco FJ, Ryan PB, Schuemie MJ, Stang PE, et al. (2018) Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes Metab 20(3): 582-589.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...