Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Review Article(ISSN: 2637-4749) 
Rift Valley Fever: An Overview with an Indian Perspective Volume 4 - Issue 1
Balvinder Kumar*, Anju Manuja, Dharvi Chhabra, BN Tripathi
- ICAR-National Research Centre on Equines, India
Received: September 08, 2020; Published: September 23, 2020
Corresponding author: Balvinder Kumar, ICAR-National Research Centre on Equines, Hisar-125001, Haryana, India
DOI: 10.32474/CDVS.2020.04.000180
Abstract
Rift Valley fever (RVF) is an important transboundary zoonotic viral disease that is endemic in Africa. Besides previous reports, the World Health Organization received reports of human deaths and abortions in RVF-affected animals in some parts of Africa in 2016. Human RVF cases in China (2016) and later in France in 2019 suggest alertness towards this viral disease, in adjoining countries. In this article, we briefly present the vital information on this disease, which will help the country for emergency preparedness to face the potential threat from RVF.
Introduction
The transmission of infectious diseases between continents and countries has become more relevant due to globalization in trade. Transboundary animal diseases (TADs) can readily spread to other countries and cause epidemics; therefore, they are of economic significance for trade for a considerable number of countries; where control/management, including exclusion, requires cooperation among several countries. Rift Valley Fever (RVF) is a zoonotic viral disease and included in list A diseases of the International Office of Epizootics (OIE) International Animal Health Code. Therefore, it is pertinent to include RVF in the list of TADs. It is also included in the listed diseases for multispecies, defined as “communicable diseases which have the potential for serious and rapid spread, irrespective of national borders; which are of serious socio-economic or public health importance; and which are of major importance in the international trade of animals and animal products.” The epidemics and epizootics of RVF are often preceded by heavy rainfalls and flooding, which create the right breeding conditions for mosquitoes, the primary vector of this virus. The outbreaks of RVF have been reported regularly in Africa, often with a devastating impact on the socio-economic situation. The reports of human RVF cases from China in 2016 and later from France in 2019 indicates vigilance towards this viral disease. China confirmed the first report of RVF in 2016 [1]. We have already pointed out the essence of transboundary preparedness of this viral disease in neighboring countries like India [2]. In India, the occurrence of antibodies to the RVF virus (or a closely related virus) in sheep and goats was first reported from Rajasthan state in 1995 [2,3], and an epidemic of RVF-like disease among sheep in Tamil Nadu state of India [4]. Here, we briefly present the important information on this disease. It will help the country for emergency preparedness to face the potential threat from RVF.
Virus and Epidemiology
The disease is caused by a virus of the family Bunyaviridae, genus Phlebovirus. RVF virus contains a single serotype and has morphological and physicochemical properties characteristic of bunyaviruses single-stranded negative-sense RNA genome. It is tripartite, the large segment (L) encodes the RNA-dependent RNA polymerase. The medium segment (M) encodes two major envelope glycoproteins, GN and GC, and a non-structural protein NSM [5]. The S-segment utilizes an ambisense strategy to encode the nucleocapsid protein and the non-structural protein, NSs, which is an interferon antagonist. The disease is endemic in Eastern Africa (Madagascar, Mozambique, Zimbabwe, Zambia, Kenya), Western Africa (Gambia, Senegal, Mauritania, Namibia), Northern Africa (Sudan, Egypt), Southern (South Africa, Namibia), Saudi Arabia, Yemen. The sporadic isolation of the virus and serologic clue in some Middle and South African countries was also reported [6]. Livestock is considered to be the amplifying and/or reservoir for human infections. Thus, outbreaks in farm animals show the way to human infections. Recently, a total of 129 human and animal RVF cases (109 animal foci (23 small ruminants and 86 bovine) from November 2018 to May 3, 2019,) have been reported in Mayotte, France [7]. Although reported human case fatality rates are generally low, but sometimes higher fatality rates (20%–40%) have been noted [8]. In August 2016, WHO acquired reports about undiscovered deaths among humans, along with death and abortion in livestock in the North-Western parts of Niger, and the areas are adjoining Mali. Table 1 listed the RVF disease report in animals and humans from 2010 to 2020. Four human RVF cases in South Africa have also been reported in 2018 [9]. RVF in 64 humans with mortality in 23 cases from August 2 to September 22, 2016, were reported in district Tchintabaraden in the Tahoua region. Wandering stockbreeders chiefly populate the area. Most of the cases are male farmers or animal breeders. In the troubled area, an outbreak with deaths and abortions is also reported among cattle and small ruminants during the same time. The risk of further disseminating the virus in an epidemic form within Niger and neighboring countries due to migration routes cannot be averted. The epidemics outside the African continent raised concerns about the potential spread of the virus to Europe and the Americas [10,11]. The high-density animal populated areas and the transhumance patterning immensely increases the risk of international spread. Figure 1 shows the RVF distribution in animals, as documented by OIE. The riverine delta systems in Pakistan and India are potential extension zones for RVF, although at a lower level of risk than those mentioned above because of their greater distance from the enzootic areas and the prevailing wind current and trade in animals [12].
Figure 1: Geographical distribution of Rift Valley Fever disease incidence in animals as documented by OIE. Countries with history of infections/serological evidence are marked red.

Animals Affected
Sheep, goats, cattle, and camels are the most RVF affected veterinary species. The indigenous African cattle breeds show marked resistance to RVF, compared with European breeds [12]. Bubalus bubalis, an Asian water buffalo, seems to be relatively resistant to RVF, but antibodies to RVF have been documented. Buffalo herds mixing with cattle exhibiting high abortion rates caused by RVF have a much lower number of abortions. Horses experience an inapparent infection with RVF. There is a short period of viremia that leads to antibody development. Birds and pigs are not affected. Pigs are relatively non-susceptible but respond with a viremia after a high titer parenteral inoculation with RVF [12]. The detection of antibodies to RVFV in 9.8% horses in Nigeria, although lower than small ruminants, suggests the possible involvement of the species in the transmission cycle of the virus [23].
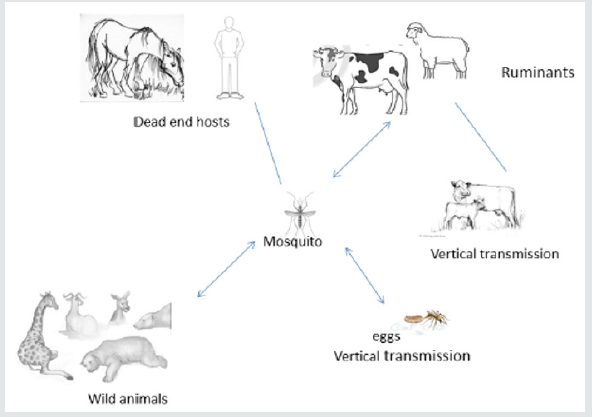
Transmission
Humans can be infected with RVFV from bites of infected
mosquitoes [9,22-28] and, rarely, from other biting insects with
virus-contaminated mouthparts. Humans are infected after
exposure to blood, body fluids, or tissues of infected animals [6,23-
27] when exposed directly during slaughter or treatment. Infection
through the aerosol transmission of RVF virus has occurred in the
laboratory environment [31]. No human-to-human transmission
has been documented. Generally, Culex and Aedes species of
mosquitoes are vectors for the RVF virus. The dominant mosquito
species vary by region, which impacts the transmission cycles
of RVFV. Environmental factors, especially rainfall, seem to be a
significant risk factor for epidemics: epizootics. The outbreaks in
humans in the flooded or heavy rainfall areas have been noticed.
RVF virus can be transmitted vertically from female mosquitoes
to the egg, where it remains viable for several years under dry
conditions. Excessive rainfall enables more mosquito eggs,
generally Aedes, to hatch and resulting in the spread of virus and
transmission to animals and humans. Epizootic events increase the
risk of exposure to infection for humans. The possible transmission
mechanism of RVFV is presented in Figure 2.
Clinical Findings
Due to non-specific clinical signs of RVF in animals, it is difficult to recognize individual cases. The incubation period in lambs is 12-36 hours with a biphasic fever (up to 42°C), anorexia, listlessness, abdominal pain, and reluctance. Icterus is a common symptom in calves. Mortality in young lambs is 90%–100%, calves 70%, whereas it is 10%–30% in adult sheep and 5%–10% in cattle. The incubation period is adult animals 24–72 hr. Clinical signs in adult animals are mostly inapparent and include fever, nasal discharge, lacrimation, diarrhea, dysgalactia, and abortion. RVF is mainly a zoonotic disease. Infected persons have either in apparent symptoms or a mild illness associated with fever and liver abnormalities since the primary site of viral replication is the hepatic tissue [32]. Generally, patients recover within two days to one week after onset of illness. In severe cases, in both animals and humans, ocular lesions, encephalitis, or severe hepatic lesions with hemorrhages are also described [33]. The inflammation of the retina is the most common complication of RVF, with permanent vision loss in approximately 1% - 10% of affected patients.
Diagnosis
Although the clinical signs of RVF are non-specific; however, the numerous abortions and mortalities among young animals, together with the influenza-like disease in humans, indicate the disease. The severity of the clinical disease varies by species and age, as described in the previous section. One should differentiate RVF from Bluetongue, Wesselsbron disease, Enterotoxemia of sheep, Ephemeral fever, Brucellosis, Vibriosis, Trichomonosis, Nairobi sheep disease, Heartwater, Ovine enzootic abortion, Toxic plants, Bacterial septicaemias, Rinderpest and Peste des petits ruminants, Anthrax. During the early phase of illness, virus isolation can detect the virus from the blood, whereas antigen-detection ELISA and PCR are the choice of assays for tissues from the infected animal collected during postmortem. ELISA can detect both IgM and IgG antibodies specific to the RVF virus in an early transient stage or later, respectively, since IgG antibodies persist for several years. Virus neutralization and haemagglutination inhibition tests are assays mentioned in the literature for RVF diagnosis. In early stages, leucopenia and a rise in enzymes associated with liver pathology, especially glutamyl dehydrogenase, are generally observed in severe clinical cases.
Prevention and Control
There is no specific treatment for RVF, and generally, symptomatic and supportive treatment is recommended. The disease’s prevention from transmitting by control of animal movements, Controls at slaughterhouses to avoid or minimize the exposure to disease, control the mosquitoes and its breeding. However, sanitary prophylaxis and vector control may have a limited effect during outbreaks. The animals may be vaccinated using either attenuated virus vaccine, inactivated virus vaccine (Smithburn strain) or Live-attenuated mutant vaccine [34-36]. The vaccine for human use is still under development [37-38]. Active surveillance for RVF is required and to detect new cases to provide timely warning and emergency preparedness for veterinary and human public health authorities.
Risk Management
We should develop the ‘targeted’ surveillance systems ‘focusing the sampling on high-risk populations in which specific, commonly known risk factors exist’. Moreover, analysis of epidemiological information, early warning systems for animal diseases, and surveillance of mosquitoes/wild reservoirs may help in monitoring of the disease [39]. Sentinel animal surveillance can function as an early warning tool. Sentinel species are different from the target species. The priority should be on identifying the first invasion of a disease or its vector into previously free regions or detecting its reemergence. Satellite remote sensing technology is the novel method available to conduct active surveillance [40]. Preventing disease introduction is more cost-effective and more straightforward than the control and elimination of an introduced pathogen. Animal quarantine and certification services (AQCS) check the entrance of animal pathogens foreign to India as per provision of Livestock importation act 1898 and amended in 2001, Sanitary and Phytosanitary Measures of the World Trade Organization (WTO, 1994) [2]. An assessment of the risk for hospital-acquired transmission of RVF was carried out in a study, and no risk was reported [41]. Risk assessment should be based on geographical distribution, wind velocity, information of disease in adjoining countries, history of introduction in-country, and quarantine procedures. Disease modeling and risk assessment by Geo-Spatial analysis of vector abundance and inclusion of disease testing in animal quarantine centers before their ingress in India will facilitate prediction and risk profiling of the RVF.
References
- Wiwanitkit V (2016) Emerging Rift Valley fever in China: What should be known? Asian Pacific Journal of Tropical Biomedicine 6(9): 727-729.
- Kumar B, Manuja A, Tripathi BN (2019) Why trans-boundary and one health preparedness for Rift Valley fever is required. Travel medicine and infectious disease 30: 139-140.
- Joshi MV, Umarani UB, Pinto BD, Joshi GD, Paranjape SP, et al. (1995) Prevalence of antibodies to Rift Valley fever (or closely related) virus in sheep and goats from Rajasthan, India: a preliminary report. Indian Journal of Virology 11(1): 59-60.
- Joshi MV, Elankumaran S, Joshi GD, Albert A, Padbidri VS, et al. (1998) A post-epizootic survey of Rift Valley Fever-like illness among sheep at Veerapuram, Chennai, Tamil Nadu. Indian J Virol 14: 155-157.
- Wilson WC, Davis AS, Gaudreault NN, Faburay B, Trujillo JD, et al. (2016) Experimental infection of calves by two genetically distinct strains of Rift Valley fever virus. Viruses 8(5): 145.
- http://www.searo.who.int/entity/emerging_diseases/Rift_Valley_Fever
- https://www.who.int/csr/don/13-may-2019-rift-valley-fever-mayotte-france/en
- Nanyingi MO, Munyua P, Kiama SG, Muchemi GM, Thumbi SM, et al. (2015) A systematic review of Rift Valley fever epidemiology 1931–2014. Infect Ecol Epidemiol 5(1): 28024.
- Jansen van Vuren P, Kgaladi J, Patharoo V, Ohaebosim P, Msimang V, et al. (2018) Human Cases of Rift Valley Fever in South Africa, 2018. Vector-Borne and Zoonotic Diseases 18(12): 713-715.
- Chevalier V (2013) Relevance of Rift Valley fever to public health in the European Union. Clin Microbiol Infect 19(8): 705–708.
- Rolin AI, Berrang Ford LKMA (2013) The risk of Rift Valley fever virus introduction and establishment inthe United States and European Union. Emerg Microb Infect 2(12): e81.
- http://www.fao.org/docrep/006/y4611e/y4611e02.htm
- (2016) WHO Disease Outbreak News.
- Boushab BM, Fall-Malick FZ, Baba O, El Wafi S, Ould Salem ML, et al. (2016) Severe human illness caused by Rift Valley Fever virus in Mauritania, 2015. In Open forum infectious diseases Oxford University Press, USA 3(4).
- Bob NS, Bâ H, Fall G, Ishagh E, Diallo MY, et al. (2017) Detection of the Northeastern African Rift Valley Fever Virus Lineage during the 2015 Outbreak in Mauritania. In Open Forum Infectious Diseases, Oxford University Press, USA 4(2).
- Sow A, Faye O, Ba Y, Diallo D, Fall G, et al. (2016) Widespread Rift Valley fever emergence in Senegal in 2013–2014. In Open forum infectious diseases, Oxford University Press, USA 3(3).
- Sow A, Faye O, Ba Y, Ba H, Diallo D, et al. (2014) Rift Valley fever outbreak, southern Mauritania, 2012. Emerging infectious diseases 20(2): 296-299.
- Archer BN, Thomas J, Weyer J, Cengimbo A, Landoh DE, et al. (2013) Epidemiologic investigations into outbreaks of Rift Valley fever in humans, South Africa, 2008–2011. Emerging infectious diseases 19(12): 1918-1925.
- Métras R, Porphyre T, Pfeiffer DU, Kemp A, Thompson PN, et al. (2012) Exploratory space-time analyses of Rift Valley fever in South Africa in 2008–2011. PLoS neglected tropical diseases 6(8): e1808.
- Faye O, Ba H, Ba Y, Freire CC, Faye O, et al. (2014) Reemergence of rift valley Fever, mauritania, 2010. Emerging infectious diseases 20(2): 300-303.
- El Mamy AB, Baba MO, Barry Y, Isselmou K, Dia ML, et al. (2011) Unexpected Rift Valley fever outbreak, northern Mauritania. Emerging Infectious Diseases 17(10): 1894-1896.
- Monaco F, Pinoni C, Cosseddu GM, Khaiseb S, Calistri P, et al. (2013) Rift Valley fever in Namibia, 2010. Emerging infectious diseases 19(12): 2025-2027.
- Olaleye OD, Tomori O, Schmitz H (1996) Rift Valley fever in Nigeria: infections in domestic animals. Revue scientifique et technique. International Office of Epizootics 15(3): 937-946.
- Ganter M (2015) Zoonotic risks from small ruminants. Vet Microbiol 181(1–2): 53-65.
- Baba M, Masiga DK, Sang R, Villinger J (2016) Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg Microbes Infect 5(6): e58.
- Mansfield KL, Banyard AC, McElhinney L, Johnson N, Horton DL, et al. (2015) Rift Valley fever virus: are view of diagnosis and vaccination, and implications for emergence in Europe. Vaccine 33(42): 5520-5531.
- Himeidan YE, Kweka EJ, Mahgoub MM, El Rayah ElA, Ouma JO (2014) Recent outbreaks of Rift Valley fever in East Africa and the Middle East. Front Public Health 2: 169.
- Tantely LM, Boyer S, Fontenille D (2015) A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am J Trop Med Hyg 92(4): 722-729.
- LinthicumKJ, Britch SC, Anyamba A (2016) Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol 61: 395-415.
- Bird BH, McElroy AK (2016) Rift Valley fever virus: unanswered questions. Antivir Res 132: 274-280.
- https://www.cdc.gov/vhf/rvf/transmission/index.html
- Paweska JT (2015) Rift Valley fever. Rev Sci Tech 34(2): 375-389.
- Rakotoarivelo RA, Andrianasolo R, Razafimahefa SH, Randremandranto Razafimbelo NS, Randria MJ (2011) Severe presentations of Rift Valley fever in Madagascar. Med Mal Infect 41(6): 318-321.
- Gerdes GH (2004) Rift Valley fever. Rev Sci Tech 23(2): 613-623.
- Gerdes GH (2002) Rift Valley fever. Vet Clin North Am Food Anim Pract 18(3): 549-555.
- Metras R, Cavalerie L, Dommergues L, Merot P, Edmunds WJ, et al. (2016) The epidemiology of Rift Valley fever in Mayotte: insights and perspectives from 11 years of data. PLoS Negl Trop Dis 10(6): e0004783.
- Mansfield KL, Banyard AC, McElhinney L, Johnson N, Horton DL, et al. (2016) Rift Valley fever virus. A Bird BH, McElroy AK (Eds.), Rift Valley fever virus: unanswered questions. Antivir Res 132: 274-280.
- Kortekaas J (2014) One health approach to Rift Valley fever vaccine development. Antivir Res 106: 24-32.
- Martin C, Pastoret PP, Brochier B, Humblet MF, Saegerman C (2011) A survey of the transmission of infectious diseases/infections between wild and domestic ungulates in Europe. Veterinary research 42(1): 70.
- Linthicum KJ, Bailey CL, Davies FG, Tucker CJ (1987) Detection of Rift Valley fever viral activity in Kenya, by satellite remote sensing imagery. Science 235: 1656-1660.
- Al-Hamdan NA, Panackal AA, Al Bassam TH, Alrabea A, Al Hazmi M, et al. (2015) The risk of nosocomial transmission of Rift Valley fever. PLoS Negl Trop Dis 9(12): e0004314.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...