
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Relation of Dipeptide Enterocin A/P and Enterocin M with Staphylococcus aureus SA5 during Processing of Cow Milk Lump Cheese Volume 5 - Issue 2
Lauková A1* , Burdová O2 and Nagy J2
- 1Centre of Biosciences of the Slovak Academy of Sciences, Institute of Animal Physiology, Košice, Slovakia
- 2University of Veterinary Medicine and Pharmacy, Košice, Slovakia
Received: October 11, 2022; Published: October 18, 2022
Corresponding author: Andrea Lauková, Centre of Biosciences of the Slovak Academy of Sciences, Institute of Animal Physiology, Šoltésovej 4-6, 040 01 Košice, Slovakia
DOI: 10.32474/CDVS.2022.05.000207
Abstract
Microbial contamination of milk can be problem during its technological processing. Especially Staphylococcus aureus can produce enterotoxins and they can influence technological processing and health of consumers as well. Bacteriocins represent a promising approach to control bacteria during cheese processing. The aim of this study was checking relation of dipetid bacteriocin, Enterocin A/P and Enterocin M with S. aureus SA5 during processing of cow milk lump cheese. The count of controlled bacteria in reference milk vat/cheese was under detection limit. The count of SA5 strains was high in control vat (CV) and in E2 vat (SA5 strain and Ent M addition) at day 0/1, meaning that Ent M did not influence SA5 counts. However, in E1 vat (SA5 strain and Ent/A/P addition) decrease of SA5 cells was noted (difference 1.94 log cycle), which was prolonged up to day 7 (reduction from 4.1 cfu/ml/g up to 2.1 cfu/ml/g between CV and E1). At day 6 was found difference 1.0 log cycle between CV and E2. It seems, that Ent A/P showed anti-staphylococal effect, while Ent M did not possess anti-staphylococcal activity. LA and pH were not influenced by SA5 and enterocins addition. The acidity values were the highest in E1 vat at day 7 (127 ˚SH/100/ml; they were the lowest in E2 vat/cheese (90 ˚SH/100/ml).
Keywords:Cow milk lump cheese; enterocins; staphylococci; treatment
Introduction
In spite of the high nutritional qualities of bovine milk, it is also a good growth matrix for a variety of spoilage microbiota [1]. Spoilage bacteria can cause contamination in food industry [2]. In dairy, microbial contamination of milk can be problem during its technological processing. Among the most frequently appeared contaminants of raw milk belong staphylococci [1-4]. Especially Staphylococcus aureus species is the major pathogen which is often involved in foodborne outbreaks [5]. Some strains of S. aureus can produce e.g. hemolysins or enterotoxins which can influence technological processing but also health of consumers [6]. Therefore, control of these bacteria in food is essential to food safety [5]. Different physical and chemical methods have been used to control foodborne pathogens or spoilage bacteria such as pasteurization, cooking, drying, radiation, acidification, salting or addition preservatives [5]. Producers and consumers prefer mainly natural additives such as phytochemicals, and/or plant extracts. However, also bacteriocins represent natural additives. These antimicrobial proteinaceous substances are produced by some bacterial strain species and showed antimicrobial effect not only in situ or in vitro [7,8]. They also beneficially influence animals health [9] as well as safety of animal-derived food products [10]. Therefore, the aim of this study was to test effect of bacteriocins-enterocins Ent A/P and Ent M in situ - in cow milk lump cheese which were inoculated with S. aureus SA5 strain. The idea followed further possible enterocins utilization in protection of cheeses. Regarding the enterocins, mostly those enterocins produced by Enterococcus faecium strains are the best studied [11-13]. However, nowadays also enterocins produced by the other species are known [14, 15]. Ent A/P is dipeptid with a broad antimicrobial spectrum [12] which has been shown as beneficial in rabbit broilers [9]. After its administration in rabbits was noted increase in weight gain, reduction of feed conversion, phagocytic activity was stimulated and gut microbiota was optimized due to reduction of coliforms and methicillin-resistant staphylococci as well. Similarly, Ent M is a thermo-stable, small peptide with a broad antimicrobial activity [13] as well as with benefit application in poultry, broiler rabbits or horses [16-18]. Therefore, we decided for these two enterocins to be used in presented study.
Materials and Methods
Cow Milk Lump Cheese Manufacturing Process
Cow milk lump cheese was manufactured from milk in 20-L (liter) vats using the standard technology for this type of cheese as previously described by Grieger et al. [19,20].Pre-heated milk vats were divided into the reference vat/cheese (RV), the control vat/cheese CV (107 cfu/ml of Staphylococcus aureus SA5, isolated in our laboratory from mastitis milk), the experimental vat/cheese 1 (E1= inoculation with SA5 strain and dipeptid Ent A/P addition in its precipitated form (partialy purified) with its initial inhibitory activity 12 800 AU/ml against the principal indicator Enterococcus avium EA5). The experimental vat cheese 2(E2) was incoculated with SA5 strain and Ent M was added (its partial purified form-precipitate) possessing initial activity 12 800 AU/ml against EA5 strain. Then, appropriate amount of mesophilic cream culture (150 ml), rennet (20 ml) as well, and40% CaCl2 (20 ml) was added to each vat calculated for 20 L. Curds were cut (15 min), scalded, pressed, cheese lumps were formed. They were put to drop off(18-20˚ C) and cheeses were stored in cold room for 7 days. Sampling (10g) for microbiota detection was provided at day 0/1 (start of the experiment), at day 2, 4, 6 and 7. ThepHvalues, ˚SH acidity andlactic acid were checked at day 0/1, day 3, 6 and 7.Before cheese experiment in vitro inhibitory activity of Ent A/P and Ent M was checked using diffusion agar spot test [21] against SA5 strain. Inhibitory activity was expressed in AU/ml (1 600 AU/ml). The principal indicator strain E. avium EA5 (our isolate) was positive control (inhibitory activity 12 800 AU/ml)
Standard Microbial Analysis and SA5 Strain Confirmation Using PCR
Before the experiment, milk was analyzed for staphylococci using Baird-Parker agar (ISO 6888-1) with supplement and yolk tellurite (Oxoid, Ltd. Basingstoke, United Kingdom) as well as on plate count agar (pH 7,0 Biomark Laboratories, Pune, India). Bacterial counts in cheese milk were under detection limit. Then samples/or cheese homogenates (10g) were homogenized in 90 ml of sterile peptone water (Merck, Germany) treated in Stomacher-Masticator PK400, IUL (Spain). Decimal dilutions (in Ringer solution, pH 7.0) were prepared according to ISO (standard microbiological method-International Organization for Standardization). Appropriate dilutions were spread on BP agar (Oxoid) and cultivated at 30 ˚C for 48 h. Each cheese was checked in duplicate. Bacterial counts were expressed in colony forming units per gram/milliliter of milk/cheese (cfu/ml/g). SA5 strain was also confirmed using PCR with the following primers: Sau1-F,5´-TCT TCAGAA GAT GCG GAA TA-3´and Sau2-R, 5-TAA GTC AAA CGT TAA CAT ACG-3´ with 30 cycles at 58 ˚C, 30 cycles at 72 ˚C, finally 5 min at 72 ˚C according to Forsman et al. [22]. Positive control was strain ATCC25923. PCR product was visualized using 1.5% (v/w) agarose gel (420 bp) as previously described by Lauková et al. [23].
Enterocins Preparing and Their Activity
Dipeptide enterocin A/P is produced by non-autochthonous strain Enterococcus faecium EK13 which was deposited in Czech Culture Collection in Brno (Czech Republic) CCM 7419, and Ent M is a new type of enterocin produced also by non-autochthonous strain E. faecium AL41 deposited in CCM with number CCM 8558. Both strains were isolated in our laboratory. For experiment precipitate of enterocins (Ent) were prepared as previously described by Mareková et al. [12,13]. Briefly, pre-inoculum of the producer strains EK13=CCM 7419 and AL41=CCM 8558 in MRS broth (pH.7,0 Merck, Darmstadt, Germany) was inoculated into 500 ml of MRS broth and incubated overnight at 37 ˚C. Then cultures were centrifuged at 10,000 x g for 30 min. The supernatants were adjusted to have pH 5.0 (for Ent M pH 5.5) and precipitated with ammonium sulphate (40% saturation) by stirring at 4 ˚C for 4-7 h. In case of Ent M precipitation was at laboratory temperature for 1 h. Then it was centrifuged again at 10,000 x g for 30 min and precipitates were re-suspended in the minimal volume of 10 mM phosphate buffer (pH 5.0, pH 6.5 for Ent M). Bacteriocin activity was checked against the principal indicator Enterococcus avium EA5 using diffusing agar spot test [21]. Precipitates were stored at -20 ˚C until its use.
Inhibitory Activity in Cheese
Samples were treated as previously described Lauková et al. [24]. The homogenized samples (in sterile 0.1% of trisodium citrate solution) were heated at 80 ˚C for 10 min and centrifuged (10,000 x g) at 4 ˚C for 10 min. The bacteriocin/inhibitory activity was tested by the diffusion agar spot test [23] against the principal indicator E. avium EA5 as well as against S. aureus SA5. The titer of bacteriocin activity was quantified and expressed in arbitrary units (AU/ml) meaning the reciprocal of the highest sample dilution showing inhibition.
Lactic Acid, and Acidity Measurement (pH and ˚SH)
Lactic acid (LA) was measured using the isotachophoric method (YKi-001) with a detector and leading and terminating electrolytes as previously described by Lauková et al. [25]. As the leading electrolytes, 10-2 M HIS, 10-2 M HIS Cl, 0.1 % MHEC 10 mL, pH 6.0 were used, and as the terminating electrolyte 5 x 10 -3 glutaric acid and 5 x 10-3 TRIS, pH 7-9 were used. The standard was lactate calcium. The pH measurement was carried out by inserting the pin electrode of the pH meter Jenway 3310 (England) at 24 h, 72 h, and 96 h (cheese manufacturing). Acidity (˚SH/100/ml) was checked by Soxhlet-Henkel method, in ˚SH/100/ml [26].
Results and Discussion
Thebacterial count in RV was under detection limit. At day 0/1, SA5 cells count was high in control vat (CV) and in E2 (SA5/Ent M) meaning that Ent M did not start with influencing SA5 strain or no competitive relation was appeared between SA5 strain and Ent M (Table 1). On the other hand, in E1 (SA5/Ent/A/P) decrease in SA5 cell count was noticed with difference 1.94 log cycle. This decrease in SA5 cells was prolonged up the end of checking day 7. SA5 strain was reduced from 4.1 CFU/ml/g up to 2.1 cfu/ml/g with differences ranged from 1.9, 2.3 up to 2.5 log cycles between CV and E1 (Table 1). In E2 slight difference was found at day 2 between CV and E2 (difference 1.0 log cycle at day 6. It seems, that Ent A/P showed anti-staphylococal effect, while Ent M did not possess this inhibitory activity in cheese. Survived SA5 cells grown on media were confirmed by PCR.
Microbial quality of raw milk is of particular importance. Fotua et al. [27] represented that in 24% cases of controlled sheep milk S. aureus was detected. Therefore, to avoid this bacterial contamination is requested. Promising approach for these purposes represents bacteriocins. E. g. enterocin CCM 4231 showed anti-staphylococcal effect in yoghurt and also in Sunar (milk nourishment for suckling babies) as reported in our previous studies [28]. Experimentally inoculated S. aureus Oxford 209P in Sunar and SA1 in yoghurt were found reduced with difference up to 3.0 log cycles. However, direct inhibition activity of Enterocin 4231 was detected only immediately after its addition in yoghurt (400 AU/ml and after 3 and half hour (200 AU/ml) testing against the principal indicator E. avium EA5 using agar spot test. Toxinogenic S. aureus has been regularly appeared in milk in low numbers and its growth during uncontrolled fermentation of milk and/or young cheese may be intensive [29]. De Buyser et al. [29] reported that milk and cheeses were implicated in 1-5 % of the total bacterial outbreaks in foodborne diseases with the most frequently identified S. aureus as a causative agent. Therefore, utilizing antimicrobial effect of enterocins has an advantage from both processing/technological condition during products processing and also from aspect of consumers health protecting. In general, enterocins have been reported as perspective to be used in food industry [29]. However, the optimal use of bacteriocins within a multi-barrier system to inhibit spoilage microbiota requires a detailed knowledge of their nature and of those factors that may limit their effectiveness. These factors may include the food structure and composition, and the bacteriocin interaction with the other microbiota [30].
The values of LA in cheeses were not influenced by additives; these values were well balanced. However, they werehigher at day 6 and 7 in E1, E2, and CV than in RV. The pH values were also balanced in all samples with the lowest pH in E2 at day 7. The highest acidity values were in E1 (Table 2) with the highest value at day 7 (127˚ SH/100 ml), and the lowest value in E2 (90 ˚SH/100ml). The active acidity (pH) and titrable acidity (˚SH) are parameters which can influence the most ripening process during cheese processing and LA production as well [31]. However, it looks, that bacteriocins did not have impact on these parameters. Vanegas-Ortega et al. [32] even reported production of bioactive peptides from lactic acid bacteria as a sustainable approach for healthier foods. The interaction between food-derived peptides and microorganisms is very promising. Bacteriocins can be also encapsulated which can increase their stability [32]. Muruzovic et al. [33] mentionedantimicrobial effect of enterocins against a number of food pathogens meaning to use them as food protecting substances. It means, it is requested to continue in this testing to support all findigs.
Table 1: Staphylococcal count (S. aureus SA5) in milk vats and cheeses experimentally inoculated with SA5 strain treated with enterocins in log 10 cfu/ml/g.
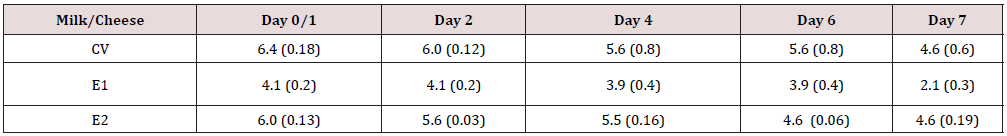
RV, Reference raw milk vat/cheese, CV-Control vat, E1, Experimental vat-inoculated with Staphylococcus aureus SA5 strain and treated with Enterocin A/P, E2, Experimental vat-inoculated with S.aureus SA5 strain and treated with Enterocin M, Before the experiment, milk was analyzed for staphylococci using Baird-Parker agar (ISO 6888-1) with supplement and yolk tellurite (Oxoid, Ltd. Basingstoke, United Kingdom) as well as on plate count agar(pH 7,0 Biomark Laboratories, Pune, India). Bacterial counts in milk for cheese production were under detection limit. Day 0/1: E1 (SA5Ent/A/P) decrease in SA5 cell count was noticed with difference 1.94 log cycle. This decrease in SA5 cells was prolonged up the end of checking day 7. SA5 strain was reduced from 4.1 cfu/ml/g up to 2.1 cfu/ml/g with differences ranged from 1.9, 2.3 up to 2.5 log cycles between CV and E1 (day 2-7). In E2 slight difference was found at day 2 between CV and E2 with difference 1.0 log cycle at day 6.
Table 2: The pH, ˚SH/100/ml and lactic acid values during processing cow milk lump cheeses inoculated with Staphylococcus aureus SA5 and treated with enterocins.
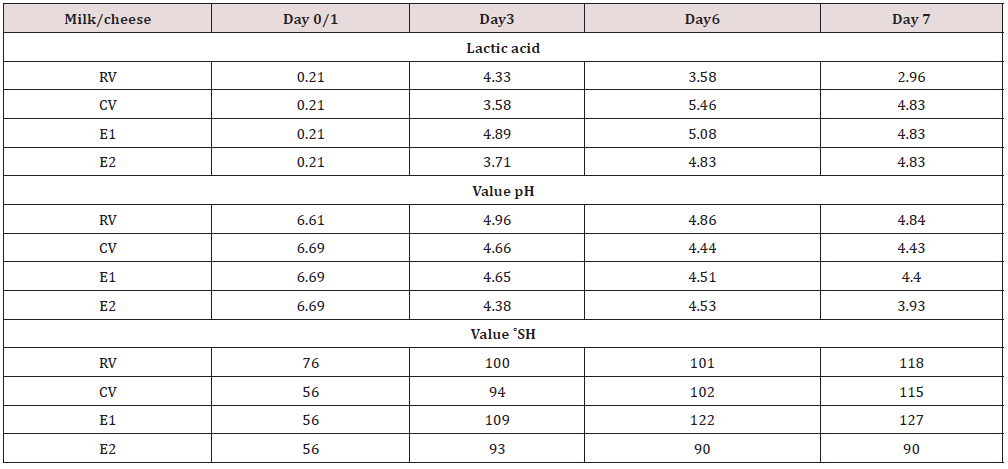
RV, Reference raw milk vat/cheese, CV-Control vat, E1, Experimental vat-inoculated with Staphylococcus aureus SA5 strain and treated with Enterocin A/P, E2, Experimental vat-inoculated with S.aureus SA5 strain and treated with Enterocin M, Lactic acid expressed in g/l, ˚SH/100/ml: Soxhlet-Henkel method [26]
Conclusion
Enterocin A/P was found to reduce S. aureus count in cow milk lump cheese with differencesup to 2.5 log cycles. On the other hand, Ent M did not possess anti-staphylococal activity. The acidity parameters (pH and ˚SH) and lactic acid production were not influenced by enterocins. In spite of prelimanary experiment, Ent A/P looks as promising additive in cheese processing to avoid contamination.
Funding
Thisresearch was partially financed by the Slovak Research and Development Agency under contract No. APVV-17-0028 and partially APVV-20-0204. We thanks Mrs. Margita Bodnárová for her laboratory work.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Slovak Research and Development Agency under contract No. APVV-17-0028 and partially APVV-20-0204.
References
- Hill B, Smythe B, Lindsay D, Shepherd J (2012) Microbiology of raw milk in New Zealand. Int J Food Microbiol 157(2): 305-308.
- Sadekuzzaman M, Yang S, Mizan MFR, Ha SD (2015) Current and recent advanced strategies for combating biofilms. Compr Rev Food Sci Food Saf 14(4): 491-509.
- Lauková A, Pogány Simonová M, Focková V, Tomáška M, Droncovský M, et al. (2022) Slovak raw goat milk as a source of variable, biofilm-forming staphylococci, and their susceptibility to lantibiotic bacteriocins. JSFA Reports 2: 40-47.
- Hein I, Rgensen HJJ, Loncarevic S, Wagner M (2005) Quantification of Staphylococcusaureus in unpasteurized bovine and caprine milk by real-time PCR. Research in Microbiology 156(4): 554-563.
- Lu Z, Dockery CHR, Crosby M, Chavarria K, Patterson B, et al. (2016) Antibacterial activities of Wasabi against Escherichia coli 0157:H7 and Staphylococcus aureus. Frontiers in Microbiology 7(53): 1403.
- Burdová O, Lauková A (2005) Reduction of biological risk of aureus presence in technological processing of some dairy products, in Slovak). Milking Process (Mliekárstvo) 36: 9-12.
- Lauková A, Vlaemynck G, Czikková S (2001) Effect of Enterocin CCM4231 on Listeriamonocytogenes in Saint-Paulin cheese. Folia Microbiologica 46: 157-160.
- Šcerbová J, Lauková A, Losasso C, Barco L (2022) Antimicrobial susceptibility to natural substances of Campylobacter jejuni and Campylobacter coli isolated from Italian poultry. Foodborne Pathog Dis 19(4): 266-271.
- Pogány Simonová M, Chrastinová L, Šcerbová J, Focková V, Plachá I, et al. (2022) Preventive potential of dipeptide Enterocin A/P on rabbit health and its effect on growth, microbiota, and immune response. Animals 12(9): 1108.
- Pogány Simonová M, Chrastinová L, Lauková A (2022) Enterocin Ent7420 and sage application as feed additives for broiler rabbits to improve meat carcass parameters and amino acid profile. Meat Science 183(1): 108656.
- Franz CHMPA, van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A (2007) Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev 31(3): 293-310.
- Mareková M, Lauková A, De Vuyst L, Skaugen M, Nes IF (2003) Partial characterization of bacteriocins produced by environmental strain Enterococcus faecium Appl Microbiol Biotetechnol 94(3): 523-530.
- Mareková M, Lauková A, Skaugen M, Nes IF (2007) Isolation and characterization of a new bacteriocin termed enterocin M, produced by environmental isolate Enterococcus faecium J Ind Microbiol Biotechnol 34(8): 533-537.
- Feng G, Guron GKP, Churey JJ, Worobo RW (2009) Characterization of mundticin L, a Class IIa anti-listerial bacteriocin from Enterococcus mundtii Appl Environ Microbiol 75(17): 5708-5713.
- Du Lihui, Somkuti GA, Renye JA, Huo Guicheng (2012) Properties of durancin GL, a new antilisterial bacteriocin produced by Enterococcus durans J Food Saf 32(1): 74-83.
- Lauková A, Strompfová V, Plachá I, Cobanová K, Faix Š, et al. (2015) Beneficial effect of enterocin-Mproducing, probiotic strain Enterococcus faecium AL41 in model experiment with hens. Glob J Anim Sci Res 3(1): 206-213.
- Lauková A, Chrastinová L, Pogány Simonová M, Strompfová V, et al. (2012) Enterococcus faecium AL41:Its Enterocin M and their beneficial use in rabbits husbandry. Probiotics and Antimicrobial Proteins 4: 243-249.
- Lauková A, Styková E, Kubašová I, Gancarcíková S, Plachá I, et al. (2018) Enterocin M and its beneficial effects in horses-a pilot experiment. Probiotics and Antimicrobial Proteins 10: 420-426.
- Grieger C, Burdová O (1978) Hygiene of Milk and Dairy products; eds. University of Veterinary Medicine, Slovakia, Europe.
- Lauková A, Burdová O, Strompfová V, Pogány Simonová M, Koréneková B (2014) Surviving of commercial probiotic strain Lactobacillus rhamnosus GG in Slovak cow lump milk cheese experimentally inoculated with Listeria innocua. J Microbiol Biotechnol Food Sci 4(1): 33-35.
- De Vuyst L, Calleawert B, Pot B (1996) Characterization of antagonistic activity of Lactobacillus amylovorus DCE47 and large-scale isolation of its bacteriocin amylovorin L471. Syst Appl Microbiol 19(1): 9-20.
- Forsman P, Tilsola-Timisjärni A, Alatossova T (1997) Identification of staphylococcal and streptococcal causes of bovine mastitis using 16S-23S rRNA spacer regions. Microbiol 143: 3491-3500.
- Lauková A, Micenková L, Grešáková L, Madarová M, Pogány Simonová M (2022) Microbiome associated with Slovak raw goat milk, trace minerals, and vitamin E content. Int J Food Sci 2022: 8.
- Lauková A, Czikková S (2001) Antagonistic effect of enterocin CCM4231 from Enterococcus faecium on bryndza, a traditional Slovak dairy product from sheep milk. Microbiol Res 156(1): 31-34.
- Lauková A, Burdová O, Nagy J (2022) In situ interaction Enterocin A/P with Staphylococcus aureus SA5 in goat milk lump cheese. Appl Sci 12: 9885.
- Hanuš O, Tomáška M, Hofericová M, Vyletelová-Klimešová M, Klapácová L, et al. (2015) Relationship between freezing point and raw ewes milk components as a possible tool for estimation of milk adulteration with added water. J Food Nut Res 54(4): 281-288.
- Fotoua K, Tzoraa A, Voidaroua CH, Alexopoulosb A, Plessasb S, et al. (2011) Isolation of microbial pathogens of subclinical mastitis from raw sheep milk of Epirus (Greece) and their role in its hygiene. Anaerobe 17(6): 315-319.
- Lauková A, Czikková S, Burdova O (1999) Anti-Staphylococcal effect of Enterocin in Sunar and Yogurt. Folia Microbiologica 44: 707-711.
- De Buyser ML, Dufour B, Maire V, Lafarge V (2001) Implication of milk and milk products in food-borne diseases in France and in different industrialized countries. Int J Food Microbiol 67(1-2): 1-17.
- Alvarez-Cisnero YM, Sáinz Espunéz TR, Wacher C, Fernandez FJ, PonceAlquieira E (2011) Enterocins: Bacteriocins with applications in the food industry. In Science against microbial pathogens: communicating current research and technological advances. Formatex, Eds. A. Mendéz-Vilas 2011: 1330-1341.
- Burdová O, Lauková A, Baranová M, Nagy J (2001) The effect of bacteriocins during experimental production of cow cottage cheese. (In Slovak). In Proceedings from International Conference Hygiena Alimentorum XXII, Milk and dairy products, Štrbské Pleso, Slovakia, Europe.
- Venegas-Ortega MG, Flores-Gallegos AC, Martinez-Hernándéz JL, Aguila CN, Nevárez-Moorillón GV (2019) Production of bioactive peptides from lactic acid bacteria: A sustainable approach for healthier foods. Compr Rev Food Sci Food Saf 18(47): 1039-1051.
- Muruzovic M, Mladenovic K, Zugic-Petrovic T, Comic L (2018) Characterization of lactic acid bacteria isolated from traditionally made Serbian cheese and evaluation of their antagonistic potential against Enterobacteriacae. Journal of Food Processses and Preservation 42(12): e13577.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




