Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Short Communication(ISSN: 2637-4749) 
Effect of Heat Treatment on Denaturation of Whey Proteins of Camel Milk Volume 4 - Issue 5
Almaz Genene*
- Ethiopian Meat and Dairy Industry Development Institute, Ethiopia
Received: August 26, 2021; Published: September 28, 2021
Corresponding author: Almaz Genene ,Ethiopian Meat and Dairy Industry Development Institute. Debre Zeit, Ethiopia
DOI: 10.32474/CDVS.2021.04.000196
Abstract
The current study was conducted at Haramaya University Dairy laboratory with the main objective of investigating how heat treatment affects whey proteins and rennetability property of camel milk for cheese making. Completely randomized design (CRD) was used by evaluating effect temperature (650C/30min,720C/30 sec, 750C/5 min, 850C/5 min and 900C/5 min).Raw milk used for reference. Similar experimental setup was also used for cow milk.. Heat treatment was done in thermostatically controlled water bath. The chemical composition of milk analysed using milkoscan, whey protein denaturation analysis was done using gel electrophoresis. Heat treatment have no significant effect (P>0.05) on gross chemical content of camel and cow milk. In camel milk α-lactalbumin (α-La)showed less denaturation while Camel serum albumin denatured at higher heat treatment as band become invisible. While in cow milk β-lactoglobulin and α-La denatured as temperature level increased while bovine serum albumin the denaturation percentage increased constantly as temperature increased.
Keywords:Camel Milk; Protein; Denaturation; Heat Treatment; Whey Proteins
Introduction
Camels (Camelus dromedarius) are the most important domesticated animals mainly in arid and semiarid areas of tropical and sub-tropical countries (Al haj and Al Kanhal, 2010; Hattem et al., 2011;Gerosa and Skoet, 2012) [1,2, 3]. Camel milk contains all essential nutrients as bovine milk except some difference in its chemical composition and processing property (Farah, 1993 and Marawa et al., 2013) [4,]. Whey proteins of camel milk are relatively more heat resistant than cow’s and buffalo’s milk (El-Agamy, 2000) [5]. However; camel milk is poor in heat stability as, it coagulates within less than 1 min at high temperature 1300C or 1400C that widely used for bovine milk heat stability indication. This might expected to be due to lack of the whey protein β-lactoglobulin (β- lg) and deficiency in κ-casein in camel milk since they have greatest impact on the heat stability of bovine milk (El-Agamy, 2000; El haj and Freigoun, 2015; Felfuol et al., 2016; Hailu et al., 2016) [5,6, 7,8]. Heat treatments of camel milk can improve the microbial quality and also important to extended its shelf life (Mohamed and El Zubeir, 2014) [9]. Denaturation of whey protein in milk mainly occurs during heating especially at temperatureabove 60℃ (Singh and Waungana, 2001) [10]. Heat treatment cause unfolding of whey proteins leading to exposure of hydrophobic regions and side chain groups formerly buried in the native structure, like reactive thiol groups.
[4, 11] knowledge denaturation of individual whey protein can provide optimization of milk heat treatment temperature. Therefore this study was conducted main objectives to investigate the effect of different level of heat treatment on whey proteins or denaturation of camel milk.
Materials And Methods
Material
Fresh Camel milk sample from dromedary camel was bought from the pastoralist in Ererr valley while cow milk from Haramaya University Dairy farm was milk used for the experiment. Morning milk was collected after being pooled together in clean stainless steel containers. Seven litre of milk was collated in two times for camel milk and the same volume also collected for cow milk. Then the milk samples were transported to Haramaya University Dairy Laboratory (HUDL) using an icebox .then immediately refrigerated at 40C until subdivide for different heat treatments. MinProtean TGXstain-free precasted gels, Tris/glycine/SDS running buffer (TG10x), laemmli sample buffer, Beta-mercaptoethanol (BME) and broad range precision plus Protein Standard (Protein™ Unstained) from BIO-RAD (USA) were chemicals used for SDS-PAGE analysis. Double distilled was also used for dilution chymosin while deionized water SDS -PAGE analysis.
Experimental Procedure
Heat Treatment of Milk
After raw milk samples were analysed for chemical composition and pH using milkoscan FT1 (Foss Electric, Hillerød, Denmark) and digital pH meter, then 250 ml milk sample was filled in to 6 bottle and randomly assigned for Raw milk, heat treatment at different temperature 65°C for 30 min,72°C for 30 sec, 75°C for 5 min, 85°Cfor 5 min and 90°C for 5 min . Raw milk sample used as reference. In similar experimental setup bovine milk was also heat treated for reference. Therefore; a total of 12 treatments (6 for camel milk and 6 for cow milk) were prepared in duplication of the same experiment. Then the samples were heat treated using thermostatically controlled water bath (model memmert) according to El-Agamy et al, [5] by adjusting temperature level for each sample. Milk samples temperature was monitored using thermometer. After the desired temperature combination was attained heating was stopped. Then portion of milk samples were taken for chemical composition analysis while the rest milk samples stored at 40C for whey protein analysis.
Physicochemical Analysis
The gross chemical composition of milk such percentage of Fat, protein, lactose, total solid, solids-not-fat, casein number and lactic acid and density were measured using automatic milk analyser milkoscan FT1(Model MilkoScan™ FT1- FOSS, Hillerød, Denmark) and for this two solution were used for automatic cleaning of the instrument as indicated in the guideline of the instrument. While pH of the samples was measured using digital pH meter after calibration at 4 and 7 pH solution. The measurements for both fresh and heat treated milk samples were done by taking 80 ml milk samples from each treatment.
Gel -Electrophoresis
The denaturation level of individual whey proteins (β-Lg, α-La, serum albumin, lactoferrin ) were determined using Sodium Dodecylsulphate-polyacrylamid Gelelectrophoresis (SDS-PAGE) according to the procedure stated by Hailu et al, [12] using a vertical gel electrophoresis BIO-RAD. Milk samples were taken from heat treated and raw milk for preparation of whey protein samples by precipitation of casein at its PI at pH 4.3 for camel milk and at pH 4.6 for cow milk using1N HCl (Wangoh etal., 1998) [13]. Centrifugation was done at 4000 gravitational force (g) for 15 min using centrifuge (Model Sigma, Germany ) to remove the fat and re centrifuged to get clear acid-whey sample that was free from casein as per the method described by Omar et al, [14]. Finally a clear supernatant whey protein was extracted using disposable syringe in endorphins tubes.
Min Protean TGX stain-free precast gel having 15 μl well size, Tris/glycine/SDS running buffer (TG 10x), laemmli sample buffer, beta-mercaptoethanol (BME) and broad range precision plus Protein Standard (Protein™ Unstained) were chemicals used. All instrument and chemical were from BIO-RAD (USA). The dilution of chemicals and preparation of solution were done according to the instruction from the manufactures while de-ionized water prepared at Haramaya University.
One to one ratio of whey protein precipitated sample and sample and sample buffer with 5% BME were mixed (20:20 μL). And denatured at 900C for 5min as according to El agamy et al, [5]. Then by assembling MinProteanTGXstain-free precast gels on the electrophoresis running buffer was filed in buffer dam up to the level then 15μl mixed sample were loaded using sample loading micropipette per lane and electrophoresis was run at 200 voltage for 30 min until the dye rich the end of gel. Finally, the gel was visualised using gel Doc™ EZ imager BIO-RED (gel imager). This analysis was done in Animal Genetics and Breading Laboratory of Haramaya University. The data was extracted in figure from the software output. The location of each whey protein on the band was compared with the bands of standard and previous literatures for accuracy.
Experimental Design
The experiment was conducted using a complete randomized design with six treatments of pooled milk (250 ml each). (Raw, 650C, 720C, 750C, 850C and 950C) were assigned as 6 treatments. Raw milk used as reference. In similar experimental set up cow milk also studied for reference. The experiment was done in duplicate following the same steps for both milk sources.
Statistical Analysis
The experimental data was analysed using Analysis of Variance (ANOVA) with Statistical Analysis System (SAS) 2009 version 9.2 (SAS Institute Inc., Cary, NC USA ).The data were expressed as the means ± standard deviation of values obtained from duplicates of the experiments. Statistically significant differences at (P<0.05) between mean of different treatment levels were determined by least significant difference (LSD).
Result and Discussion
Physicochemical Properties of Fresh Milk
The physic-chemical properties of raw dromedary camel and cow milk samples were indicated (Table 1).
Mean value with same superscripts letter in the same row are not significantly different at P<0.05. TS: Total Solid, SNF: Solid Non- Fat.
Effect of Heat Treatment on Camel Milk Composition
The effect of heat treatment on the major chemical composition of milk that treated at indicated temperatures was analysed and summarized based on this the % of fat, protein, lactose, and total solid of camel milk were not significantly affected (P>0.05).
Effects of heat treatment on individual whey proteins of camel and cow milk
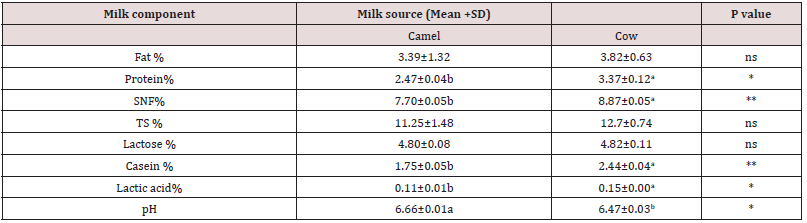
The effect of heat treatment on individual whey proteins was identified using SDS-PAGE. This was done through visual observation at the band of each fraction of whey protein based on their migration which in turn depends on their molecular weight. Figure 1 shows the denaturation level of individual whey protein at different heat treatment temperature level for both camel and bovine milk including the unheated milk that used as a reference.
The position of whey protein on the band was indicated by comparing the molecular weight of whey proteins with the protein standard and with previous literature (Farah, 1986; Hinz et al., 2012 and omar et al., 2016) [11,14, 15].
Figure 1: SDS-PAGE of whey proteins of heated camel and cow milk at different time temperature combination. NB: Whey proteins of cow milk at lane 2, 3,4,5,6,7 stands for camel milk while lane number 8, 9,10,11,12 and 13 stands for cow milk . At Lane number 1and 14 are precision plus protein Standard with molecular weight of 10-250 KDa . KDa= kilo Dalton.
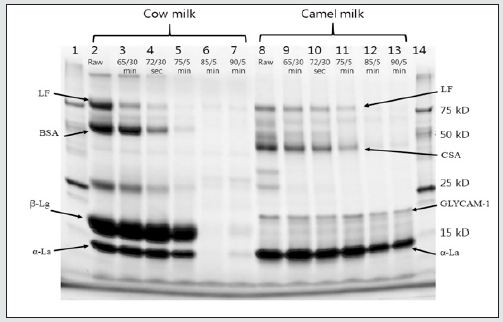
Whey protein move through the gel electrophoresis according to their molecular weight. In bovine milk β-Lg the dominate whey protein [4,11,14] indicted from lane number 2 to 7 in second lower band. The intensity of β-Lg band in cow milk seen less change at standard pasteurization however, increases at temperature to 750C the band intensity decreases even also invisible at lane 6 and 7 at higher heat treatments 850C and 900C. As compared to the band intensity percentage of β-Lg in raw milk in heat treated the band intensity level decreased. For instance, at 750C it was 64% with 36% denaturation while at 900C band intensity decreased or denaturation by 96% figure 2A. However, from the current electrophoresis analysis any band that related to β-Lg was not seen clearly for camel milk neither in raw nor for heat treated samples in lane 8 to13. This agree with different literatures that report no β-Lg in camel milk [6, 11, 14, 16].
In cow milk α-La band intensity also reduced as heat treatment level increases and even become invisible at lane 6 and 7. Band percentage of α-La of cow milk (Figure 2B) 94%, 82%, 45%,1% and 4% at 650C 720C,750C,850C and 900C, respectively as compared to reference milk sample percentages with a reduction of 6%, 18%, 55%, 99 % and 96% respectively. This might be due to availability of β-Lg in bovine milk that helps interaction of α-La with casein micelles or other whey protein (Hessey, 2011) [17]. While in camel milk (Figure 1) α-La band seen less visible change as heat treatment level increases. Even though there was some variation, the band percentage of α-la was 67% at 900C with fewer reductions of 33 % (Figure 2A) as compared to the reference milk band percentage. This might be due to lack of β-Lg in camel milk that leads less interaction with other protein.
Figure 2: Band intensity% of β-lg, α-al, CSA/BSA, Lacoferin and Glycam-1 in heat treated Camel (A) and Cow milk (B) as compared to the references milk.
Decreased in band intensity of β-Lg and α-La attribute of denaturation and interaction with casein micelles or with other whey proteins due to heat-treatment of milk at temperatures above 60°C that leads to denaturation (unfolding) of the whey proteins mainly β-lactoglobulin, followed by aggregation through hydrophobic interaction and disulphide-thiol interchanges to form heat-induced aggregates, either on the surface of the casein micelles (micelle bound aggregates), or in the serum phase of the milk (serum aggregates) depend on heating temperature and time [10,18-20].
The mechanism of association denatured whey protein with casein micelles in camel milk is not known clearly because of lack of β-Lg. However; according to Felfoul et al, [16] denatured α-La and Camel serum albumin (CSA) is able to adhere on the hot surfaces alone or interacting with the casein micelles mainly κ-casein as β-Lg was responsible for cow milk. From this point of view in the current result of electrophoresis CSA looks responsible for interaction with the ĸ- casein in camel milk during heat treatment than α-la due to less denaturetion of α-la as temperature increases. Camel serum albumin band intensity continually decreased as heat treatment increased and even invisible after 850C (Figure 2A). The band intensity reduction of CSA as compared to the references sample and the reduction was 8, 24, 58, 89 and 92% at 65,72 ,75,85 and 900C, respectively. Similarly in cow milk BSA band show continual decrease as heat treatment level increased and disappeared after 750C with reduction percentage 14 %, 64%, 93%, 99% 100% at 650C,720C,750C, 850Cand 900C, respectively as compared to % of reference milk reduction. The reduction in band intensity both in CSA and BSA supported by the literature that shows serum albumin was faster to denature [21].
For camel milk the band of lactoferrin band was visible for reference milk however the intensity of the band deceases as heat treatment increases (Figure 2). The band intensity percentage of camel milk LF was 67%, 53%, 28% ,6% and 5% at 65,72, 75 ,85 and 900C respectively compared to reference milk sample % with a reduced of 33%, 47%, 72%, 94% as increasing order of heat treatment. The band of LF in cow’s milk also decreased as level of heat treatment increases. However, looks more than what is seen in camel milk since pronounced after 750C whereas for cow milk it looks after 720C. It have been reported that BSA and LF form complex to a minor extent with αs2-casein through thiol/disulphide exchanges while Immunoglobulin partially associate through hydrophobic interactions only [21]. Therefore denatured bovine serum albumin and LF might be attached to micelles or other whey protein and that could be why their band become less visible as heating treatment increased. Denaturation rate of individual whey protein agree with the literature that indicates the thermal stability order serum albumin, β-Lg and α-La, respectively [10, 21].
The trend of β-Lg and α-La in bovine milk agree with previous work of Farah et al, [15]. As indicated on report of El-Algamy, increasing temperature to 75°C resulted visible changes on whey protein of bovine, buffalos and camel milk even though observe less denaturation of α-La in camel milk this finding supports the results from the current study. On the other hand the current SDSPAGE results at middle lower band of camel milk visible band was seen around 22-23kda. This might be GLYCAM-1 (Glycosylation- Dependent Cell Adhesion Molecule 1). On the other hand Farah et al, [15] found a new band that had estimated molecular weight of 23KDa nearly in similar band position of the current study [22-25]. However, further study about its property during heat treatment is important due to limited references regarding these whey proteins [26-28].
Conclusion
Heat treatment of milk resulted in denaturation of whey proteins depending on the intensity of heat applied both for camel and bovine milk. In camel milk, α-La showed less denaturation than bovine milk, while CSA and LF denatured as a function of increasing heat treatment level. β-Lg was not found in camel milk unlike for bovine milk. In bovine milk, both β-Lg and α-La denatured as heat treatment increased.
Recommendation
a) Camel milk heat treatment is possible without any significant effects on the gross chemical composition. This could be un advantages to reduce risk related to raw milk consumption through awareness creation by the concerned organization on importance and possibility of camel milk heat treatment to pastoralists society.
b) Further research should be done on property of denatured whey proteins of camel milk to known in detail the complex formation during heat treatment.
Acknowledgement
The author very grateful thanks to Haramaya University for providing the laboratory facilities and to the Danish International Development Agency for the necessary financial support via the Haramaya Camel Dairy project.
References
- Al Haj OA , Al Kanhal HA (2010) Compositional, technological and nutritional aspects of dromedary camel milk. International Dairy Journal 20(12): 811–821.
- Hattem HE, Manal AN, Hanna SS, Elham AA (2011) A Study on the effect of
thermal treatment on composition and same properties of camel milk. Slovak Journal of Animal Science 44: 97-102. - Gerosa S , Skoet J (2012) Milk availability Trends in production and demand and medium-term outlook : 11- 41. In Milk and Dairy product in Human Nutrition FAO.
- Farah Z (1993) Composition and characteristics of camel milk. Journal of Dairy Research 60(4): 603–626.
- El-Agamy EI (2000) Effect of heat treatment on camel milk proteins with respect to antimicrobial factors: a comparison with cows and buffalo milk proteins. Food Chemistry 68(2): 227-232.
- Elhaj AE , Freigoun S (2015) Camel’s Milk Protein Fractionation by SDS-Polyacrylamide Gel Electrophoresis. International Journal for Agro Veterinary and Medical Sciences 9( 2 ): 41-45.
- Felfoul I ,Beaucher E, Cauty Ch, Hamadi A, Gaucheron F , et al. (2016) Deposit Generation During Camel and Cow Milk Heating: Microstructure and Chemical Composition. Food Bioprocess Technology.
- Hailu Y, Hansen EB, Seifu E, Eshetu M, Ipsen R (2016) Factors influencing the gelation and rennetabilty of camel milk using camel chymosin. International Dairy
Journal 60: 62-69. - Mohamed , El Zubeir (2014) Effect of Heat Treatment on Keeping Quality of Camel Milk . Food Science and Technology 15 : 239-245.
- Singh H , Waungana A (2001) Influence of heat treatment of milk on cheese making properties. International Dairy Journal 11(4-7): 543–551.
- Hinz K, O'Connor P M, Huppertz T, Ross R P, Kelly A L (2012) Comparison of
the principal proteins in bovine, caprine, buffalo, equine and camel milk. Journal of
Dairy Research 79(2): 185–19. - Hailu Y, Hansen E B, Seifu E, Eshetu M, Petersen M A, et al. (2018)
Rheological and sensory properties and aroma compounds formed during ripening of soft brined cheese made from camel milk. International Dairy Journal 81: 122–130. - Wangoh J, Farah Z, Puhan Z (1998) Iso-electric focusing of camel milk proteins. International Dairy Journal 8(7): 617–621.
- Omar A, Harbourne N, Oruna Concha MJ (2016) Quantification of major camel
milk proteins by capillary electrophoresis. International Dairy Journal 58: 31–35. - Farah Z (1986) Effect of heat treatment on whey proteins of camel milk. Milchwissenschaf, 41: 763-765.
- Felfoul I, Lopez Ch, Gaucheron F, Attia H, Ayad M (2015) Fouling Behavior of Camel and Cow Milks Under Different Heat Treatments. Food Bioprocess Technology.
- Hennessy R J (2011) Studying Milk Coagulation Kinetics With Laser Scanning Confocal Microscopy, Image Processing, And Computational Modelling. Master of science A Thesis State University, San Luis Obispo 1-75.
- Rynne Nuala M, Beresford Thomas P, Kelly Alan L, Guinee Timothy P (2004) Effect of milk pasteurization temperature and in situ whey protein denaturation on the composition, texture and heat-induced functionality of half-fat Cheddar cheese. International dairy journal 14(11) : 989-1001.
- Guyomarch F (2006) Formation of heat-induced protein aggregates in milk as a means to recover the whey protein fraction in cheese manufacture, and potential of heat-treating milk at alkaline pH values in order to keep its rennet coagulation properties. A review Lait 86(1) : 1–20.
- Li Y, Wang W (2015) Serum Protein Aggregates in the High-Heated Milk and Their Gelation Properties in Rennet-Induced Milk Gel. International Journal of Food Properties 19: 1994–2006.
- Donato L, Guyomarch F (2009) Formation and properties of the whey
protein/κ-casein complexes in heated skim milk –A review Dairy Science Technology 89: 3-29. - Dalgleish DG (2007) The casein micelle and its reactivity. Lait 87 : 385–387.
- Dissanayake M, Ramchandran L, Donkor ON, Vasiljevic T (2013) Denaturation of whey proteins as a function of heat, pH and protein concentration. International Dairy Journal 31(2): 93-99.
- El-Agmay (2009) Bioactive components in camel milk. Chapter 6 from Bioactive Components in Milk and Dairy Products (ed) Young W. Park. Wiley-Blak Well Publishing .
- El-Hatmi H, Khorchani T , Attia H (2006) Characterization and composition of camel’s (Camelus dromedaries) colostrums and milk. Microbiology and Hygiene Animal 18 : 13–17.
- Hailu Y, Hansen E B, Seifu E, Eshetu M, Ipsen R, et al. (2016) Functional
and technological properties of camel milk proteins. A review. Journal of Dairy Research 83(4): 422–429. - Hailu Y, Hansen E B, Seifu E, Eshetu M, Petersen MA , et al. (2018) Rheological and sensory properties and aroma compounds formed during ripening of soft brined cheese made from camel milk. International Dairy Journal 81: 122-130
- Hattem HE ,Manal AN , Hanna SS , Elham AA (2011) A Study on the effect of thermal treatment on composition and same Properties of camel milk. Slovak journal of Animal Science 44 (3): 97-102.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...