Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Dietary Supplementation of Rauvolfia Vomitoria Root Extract as A Phytogenic Feed Additive in Growing Rabbit Diets: Growth Performance and Caecal Microbial Population Volume 4 - Issue 2
Alagbe JO*
- Department of Animal Nutrition and Biochemistry, Sumitra Research Institute, India
Received: January 03, 2021; Published: February 02, 2021
Corresponding author: Alagbe JO, Department of Animal Nutrition and Biochemistry, Sumitra Research Institute, Gujarat, India
DOI: 10.32474/CDVS.2021.04.000184
Abstract
The objective of this study is to evaluate the dietary supplementation of Rauvolfia vomitoria root extract as a phytogenic feed additive in growing rabbit’s diets: growth performance and caecal microbial population. A total of thirty (30) weaned rabbits were divided into five treatments with three replicates per treatment of 2 rabbits per replicate in a completely randomized design. Animals in treatment 1 (control) were fed diet basal diet with no Rauvolfia vomitoria root extract (RVME) while T2, T3, T4 and T5 were fed RVME at 20 ml, 40 ml, 60 ml and 80 ml/litre. The experiment lasted for 12 weeks and other management practices were strictly observed. Data collected were used to examine the growth performance (average daily weight gain, average daily feed intake and feed conversion ratio) and caecal microbial population (Escherichia coli and lactobacilli count). Average daily weight (ADWG) was significantly (P ˂0.05) different among the treatments. Feeding rabbits RVME from 20 ml to 80 ml/litre tends to increase the average daily feed intake (ADFI) though not a significant rate (P ˃0.05). Highest mortality was recorded in T1 with (1.10 %), none was recorded in the other treatments (P ˂0.05). Lactobacilli and Escherichia coli count were influenced by the dietary treatments (P ˂0.05). Escherichia coli count significantly reduced as the level of RVME increases while increasing RVME from 20 ml to 80 ml/ litre tend to increase lactobacilli count (P ˂0.05). It was concluded that RVME contains several bioactive chemicals and could be fed to growing rabbits at 80 ml/litre without any deleterious effect on the performance and caeca microbial population of the animals. be used to bridge the gap between food safety and production. Thus, preventing environmental contamination and high case of diseases.
Keywords:Growing rabbits; rauvolfia vomitoria root; performance; phytochemicals
Introduction
Phytogenics are natural plant-based substances used effectively in animal health and nutrition to improve livestock’s health, growth, and performance [1]. They are also regarded as natural growth promoters (NGPs) as an alternative to antibiotic growth promoter (AGPs) because of the emergence of drug resistance microorganisms, side effects of antimicrobials and the harmful residual toxicity effects of drugs observed in the food chain [2,3]. Studies have also shown that the growth promoting effect of antibiotics was correlated with the decreased activity of bile salt hydrolase, an intestinal bacterium produced enzyme that exerts negative impact on the host fat digestion and utilization [4]. Scientific reports have shown that plants exhibit wide range of biological activities such as: anti-inflammatory, antioxidants, anti-bacterial, antifungal, hepatoprotective, cytotoxic, immunostimulatory, miracicidal, antiviral and cardiovascular properties [5-7]. According to [8], there are over 250, 000 species of medicinal plants globally, some are yet to be explored. Among the potential herbal plant is Rauvolfia vomitoria. Rauvolfia vomitoria an Apocynaceae, is a medicinal plant widely distributed all over the world especially in Asia and West African countries. It is a tree that grows to a height of about 15 meters and is found in most low land forest. Traditionally, the plants (leaves root and stem bark) are used for the treatment of malaria, rheumatism, hypertension, skin disease, stomach disorders and mental disorder. The plant contains several bioactive chemicals like alkaloids, flavonoids, glycosides, saponins, phenols and reducing sugars [9,10]. Root and stem bark are commonly known for their aphrodisiac, antisporic, abortive and insecticidal properties also for the antihelminthic, apercent, dysenteric, astringent, cardio tonic, diaphoretic, hypotensive, vulnerary and febrifugic potential. Rauvolfia vomitoria has numerous pharmacological properties and could be considered as an alternative to antibiotics, this will further encourage food safety and increase productivity in animals. Therefore, this experiment was carried out to evaluate the dietary supplementation of Rauvolfia vomitoria root extract as a phytogenic feed additive in growing rabbit’s diets: growth performance and caecal microbial population.
Materials and Methods
Study area
The experiment was carried out at Division of Animal Nutrition, Sumitra Research Institute, Gujarat, India during the month of April to June, 2020. Collection, identification, preparation and analysis of Rauvolfia vomitoria root extract Fresh roots from mature Rauvolfia vomitoria tree were harvested from different plants within Sumitra Research Institute Gujarat, India and authenticated by a certified crop taxonomist (Dr. Maureen Sharma). The roots thoroughly washed with running tap water to remove dirt and later cut into bits and allowed to dry under shade for 10 days to retain the bioactive chemicals in the sample. The dried samples were blended into fine powder using mortar and pestle and stored in a well labeled airtight container for further analysis. Rauvolfia vomitoria extract was prepared by soaking 200 g of the sample in 1000 litres of water in the refrigerator at 4oC for 48 hours. The mixture was filtered using Whatman No 1 filter paper to obtain the filtrate Rauvolfia vomitoria root extract (RVME). Moisture, crude protein, crude fibre, ash, ether extract and carbohydrate of the samples were determined using methods of the Official Analytical Chemist [11]. Phytochemical evaluation of tannins, alkaloids, saponins, flavonoids, phenols, oxalate, glycosides, steroids and terpenoids were estimated using methods described by [12-14]. Vitamin analysis was carried out according to the methods outlined by [15].
Animal management and feed formulation
Thirty (30) weaned rabbits of mixed breed and sex between
6-7 weeks with an average weight of 530.9 and 533.0 grams were
used for the experiment; they were sourced from an open market
in India. The animals were housed in an all-wired cage measuring
(15×12×25 cm) and equipped with feeders and drinkers. Prior
to the commencement of the experiment, pen and cages were
properly disinfected and all other biosecurity measures were
strictly observed. They were divided into five treatments with three
replicates per treatment of 2 rabbits per replicate in a completely
randomized design. Rabbits were allowed two weeks adjustment
period during which they were fed with basal diet (morning and
evening) and given prophylactic treatment with Oxytetracycline administered intramuscularly and Ivermectin given subcutaneously
adhering strictly to the package insert. Animals were fed twice daily
between 7:30 am and 3:30 pm. Fresh feed and water were provided
ad libitum and all other management practices were strictly
observed throughout the experiment which lasted for 84 days. The
basal diet was formulated to meet the nutrient requirements of
growing rabbits according to [16].
Treatment 1: Basal diet + 0 % RVME
Treatment 2: Basal diet + 20 ml/litre RVME
Treatment 3: Basal diet + 40 ml/litre RVME
Treatment 4: Basal diet + 60 ml/litre RVME
Treatment 5: Basal diet + 80 ml/litre RVME
Measurements
Feed intake (g) was determined by subtracting feed left over
from feed served, it was estimated for each of the replicate daily.
Weight gain (g) was calculated by finding the difference between
initial weight and final weight at the end of the experiment.
Average daily weight gain (ADWG) = Final body weight – Initial
body weight
Total days of the experiment
Average total feed intake (ADFI) = Feed intake
Total days of the experiment
Feed conversion ratio (FCR) = feed intake (g) ̸ weight gain (g)
Caeca microbial population
At the end of the experiment total caecal bacterial population was determined according to the method outlined by American Public Health Association (APHA). Isolation of E. coli and lactobacilli bacteria was carried out by method described by.
Statistical analysis
All data were subjected to one -way analysis of variance (ANOVA) using SPSS (18.0) and significant means were separated using Duncan multiple range tests. Significant was declared if P ≤ 0.05.
Results and Discussion
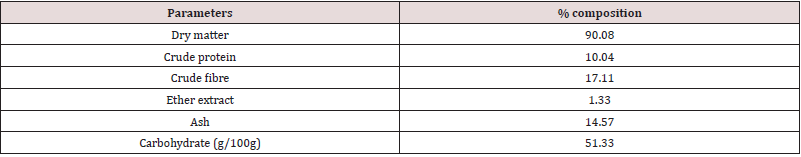
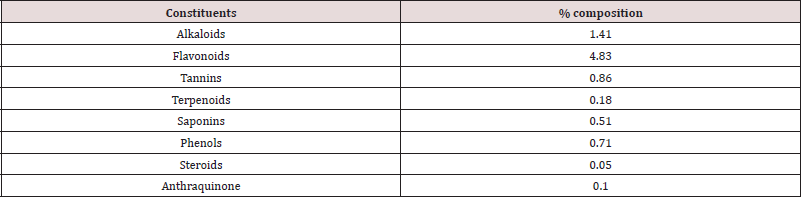
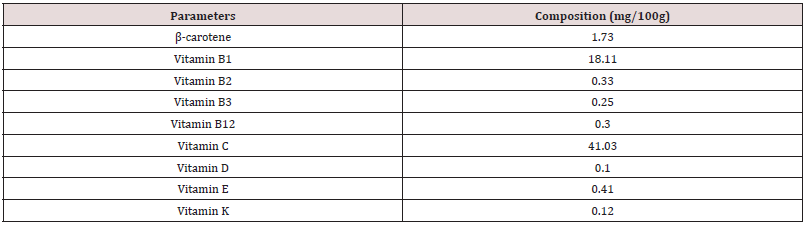
Table 1 reveals the percentage composition of experimental diet. The feed contained crude protein (17.08 %), crude fibre (9.87 %), ether extract (3.52 %), calcium (0.61 %), phosphorus (0.33 %) and energy (2500.1 kcal/kg). This conforms to the findings of [17]. The crude protein and crude fibre are with the recommended range by [18]. Ether extract fall within the recommended ranges by [19]. The energy value is in close agreement with the findings of [20] who examined the effect of neem leaf (Azadirachta indica) meal on the growth performance of rabbits. However, the proximate values of the experimental diets were within the nutritional requirement of rabbits according to [16,21]. Inadequate energy, protein or micronutrients in the diet may impair performance of rabbits [22]. A balanced diet containing prerequisites amounts of energy, proteins, fat, minerals, vitamins and water are essential for rabbits reared under intensive production [18]. Adequate fibre in the diet of animals lowers serum cholesterol level, aids digestion and prevents the risk of coronary heart disease. Adequate intake of dietary ether extracts or fats provides energy, improves palatability and transport of fat-soluble vitamins in the body [23-25]. Proteins play a central role in cell growth and strengthening the immune system [26-28]. Energy is required by rabbits for the contraction of muscles which enable the rabbit to move, build up their physiology and make products such as fur, milk and meat etc [21]. The proximate composition of Rauvolfia vomitoria root is presented in Table 2. The sample contained dry matter, crude protein, crude fibre, ash and carbohydrates at 90.08 %, 10.04 %, 17.11 %, 1.33 %, 14.57 % and 51.33 g/100g respectively. The crude protein obtained in Rauvolfia vomitoria root is lower than the values reported for Pentadiplandra brazzeama (11.30 %), Piper umbellatum (11.30 %), Scoradophleus zenkeri (12.2 %) and Solanum melongena (14.0 %) reported by [29]. However, the protein value in Rauvolfia vomitoria is low, it is an indication that it cannot be used as a protein supplement in the diet of animals [30]. Crude fibre value obtained was lower than the values reported for Momordica charantia (51.38 %) and Morinda lucida stem bark (53.49 %) reported by [31], but in agreement with the reports of [32] who reported a value of 17.00 % in Boerhavia erecta roots. According to [33], ash is an index used to determine the mineral content in a sample. The ash and ether extract values obtained in this study are in conformity with the values obtained by [32] for Boerhavia diffusa leaves (14.56 %). The result on ash is a clear indication that the sample contains enough minerals for the activities of enzymes, regulation of water, electrolyte and acid-base balance in the body, as well as responsible for nerve action and functioning of the muscles [34]. Total carbohydrate value obtained is higher than the values for Lepidium sativum (19.5 g/100g), Salvia officinalis (33.0 g/100g), Thymus capitatus (50.7 g/100g) and Hibiscus sabdariffa (19.5 g/100g) but lower than values of Corianderum sativum (63.0 g/100g) and Trigonella foenum-graecum (54.2 g/100g) reported by Ereifej et al. (2012). Phytochemical composition of Rauvolfia vomitoria root is presented in Table 3. The phytochemical result contains alkaloids (1.41 %), flavonoids (4.83 %), tannins (0.86 %), terpenoids (0.18 %), saponins (0.51 %), phenols (0.71 %), steroids (0.05 %) and anthraquinone (0.10 %). In order of abundance flavonoids ˃ alkaloids ˃ tannins ˃ phenols ˃ saponins ˃ terpenoids ˃ anthraquinone ˃ steroids respectively. The result obtained are in conformity with the values obtained by [32,35]. Phytochemicals are natural bioactive compounds that are derived from plants and incorporated into livestock feed to enhance productivity [36]. The main bioactive compounds of phytochemicals are polyphenols and their composition and concentration vary according to geographical location, parts of the plant, harvesting season, storage conditions, processing methods and environmental factors [36,37]. This bioactive chemical confers plants the ability to perform multiple biological activities such as: anti-inflammatory, antiviral, antifungal, hypolipidemic, antioxidants, anti-allergic, hepato-protective and neuro-protective. However, the values were below lethal dose levels reported by [38] who evaluated the effects of feeding different levels of Indigofera zollingeriana to growing rabbits. Table 4 shows the vitamin composition of Rauvolfia vomitoria root. The sample contains β-carotene, vitamin B1, vitamin B2, vitamin B3, vitamin B12, vitamin C, vitamin D, vitamin E and vitamin K at 1.73mg/100g, 18.11 mg/100g, 0.33 mg/100g, 0.25 mg/100g, 0.30 mg/100g, 41.03mg/100g, 0.10 mg/100g, 0.41 mg/100g and 0.12 mg/100g respectively. In order of abundance vitamin C ˃ vitamin B1 ˃ β-carotene ˃ vitamin E ˃ vitamin B2 ˃ vitamin B12 ˃ vitamin B3 ˃ vitamin K ˃ vitamin D. Vitamins are direly important for human health, growth, development, reproduction and maintenance, and their deficiencies are imposing serious health hazards [39]. They are diverse in nature relative to fats, carbohydrates and proteins and differentiated from other groups by their organic nature and their classification depends on chemical nature and function [40]. The high concentration of vitamin C is an indication that the sample will effectively perform the role of antioxidants, thus scavenging free radicals [41,42]. Vitamin B1 is essential for the normal functioning of the nervous system, digestive system and brain [43]. β-carotene are precursors of vitamin A and the provide animals with good vision, support to immune system and inflammatory systems, cell growth and development, antioxidant activity, promoting proper cell communication [44]. Vitamin E protects the membrane fats from oxidative damage and maintains the cellular functioning. This vitamin also protects the food from oxidative damage during storage and processing [44]. Vitamin B2 provides antioxidative protection and promotes iron metabolism in the body [45]. B12 vitamin also plays important role in energy metabolism and other biological processes while B3 is responsible for antioxidative defense [38]. Vitamin K is key in blood clotting while vitamin D is important for normal body functioning [46-48]. Table 5 reveals the performance characteristics of growing rabbits fed different levels of Rauvolfia vomitoria root. Initial body weight (IBW), final body weight (FBW), final weight gain (FWG) and average daily weight gain (ADWG) ranges between 530.1 – 533.0 g, 997.6 – 1331.6 g, 466.7 – 801.5 g and 5.59 – 9.51 g respectively. IBW, FBW, FWG and ADWG were highest in T4, T5, intermediate in T2, T3 and lowest in T1 (P˂0.05). Total feed intake (TFI), average daily feed intake (ADFI), feed conversion ratio (FCR) ranged between 8800.2 – 8918.0 g, 104.8 – 106.2 g and 5.99 – 9.34 respectively. TFI increased from T1 to T5, though not at a significant rate (P˃0.05). FCR and mortality were significantly different among the treatment (P˂0.05). Highest mortality was recorded in T1 (1.10 %), none was recorded in the other treatments. The results on weight gain in this study are in close agreement with the [49,16] when growing rabbits were fed diets containing foliage of browse trees. Higher weight gain in rabbits fed T4 and T5 could be attributed to the presence of phytochemicals in Rauvolfia vomitoria root. According to [50,51], bioactive chemicals in feed is capable of promoting flavor, secreting of digestive fluids and total feed intake, thus promoting better performance. Medicinal plants could also act as modifiers of metabolic activities [52-54], herbal plants are capable of performing antimicrobial role, reducing growth depressing metabolites and inhibiting the production and excretion of cytokines by immune cells, this could be the reason why mortality was not recorded in T2, T3, T4 and T5. Caecal microbial population of weaner rabbits fed different levels of Rauvolfia vomitoria root is presented in Table 6. Escherichia coli and lactobacilli count ranged between 25.01 – 42.10 (Cfu/g) and 27.88 – 50.02 (Cfu/g) respectively. E. coli and lactobacilli count were significantly (P˂0.05) different among the treatments. According to [46], various phytochemicals exhibit a wide spectrum of antibacterial activities against Gram – positive and Gram – negative bacteria. Bioactive chemicals in plants are also capable of eliminating pathogenic organisms via: direct production antagonism via production of antimicrobials, competitive exclusion through occupation of specific binding sites, stimulation of the immune response resulting in host – exclusion of the pathogen, competition for nutrients that limit microbial growth and enhanced gut health through restoration of epithelial integrity [55]. Flavonoids are capable of acting as an antifungal and antioxidants, thus scavenging free radicals and preventing diseases [56-58]. Tannins are known to possess both antibacterial and anti-viral activity [59]. Alkaloids are the most efficient therapeutically significant plant substances commonly found to have antimicrobial properties due to their ability to intercalate DNA of the microorganisms [60]. Phenols are strong antioxidants while saponins are involved in anti-inflammatory activities [61,62]. However, the results obtained in this study is in agreement with the findings of [63-65] who examined the improvement of intestinal microflora balance in animals by green tea extracts.
Table 1: Percentage composition of experimental diet,* Premix supplied per kg diet :- Vit A, 7,000 I.U; Vit E, 5mg; Vit D3, 3000I.U, Vit K, 3mg; Vit B2, 5.5mg; Niacin, 25mg ; Vit B12, 16mg ; Choline chloride, 120mg ; Mn, 5.2mg ; Zn, 25mg ; Cu, 2.6g ; Folic acid, 2mg ; Fe, 5g ; Pantothenic acid, 10mg ; Biotin, 30.5g ; Antioxidant, 56mg.
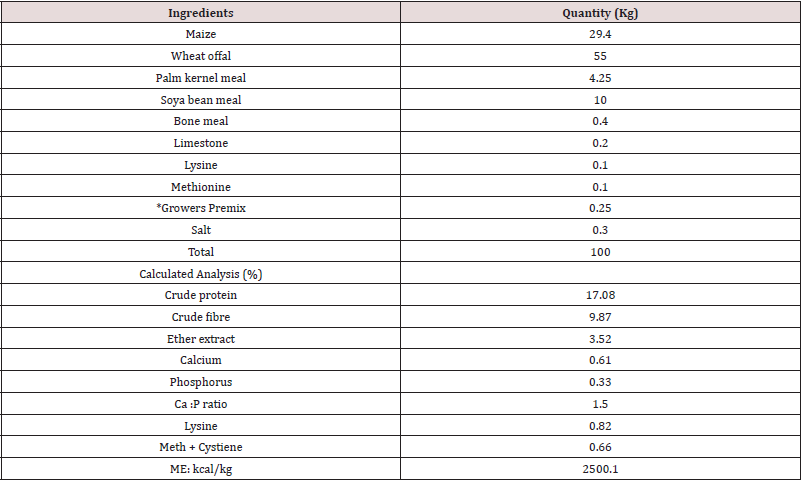
Table 5: Performance characteristics of rabbits fed different levels of Rauvolfia vomitoria root, IBW: Initial body weight; FBW: final body weight; FWG: final weight gain; ADWG: Average daily weight gain; TFI: total feed intake; ADFI: Average daily feed intake; FCR: feed conversion ratio; SEM: Standard error of mean.
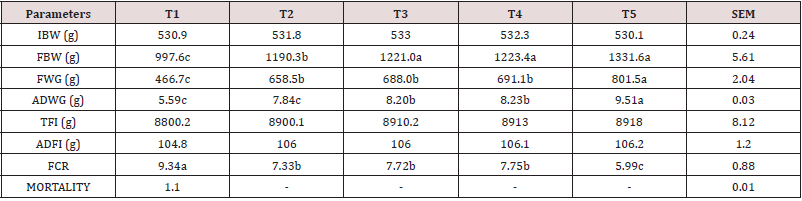
Table 6: Caecal microbial population of weaner rabbits fed different levels of Rauvolfia vomitoria root.

Conclusion
The use of Rauvolfia vomitoria root is capable of promoting food safety and preventing various ailments in human and animals. It is a cheaper alternative because it contains several bioactive chemicals such as alkaloids, flavonoids, glycosides, tannins, terpenoids, phenols etc [66-74]. These constituents at below lethal dose are considered safe, effective and relatively cheap. Rauvolfia vomitoria root extract have proven to perform multiple biological roles especially when fed to weaner rabbits at 80 ml/ litre concentration. It was concluded that feeding animals at this concentration enhanced feed intake, growth performance and was able to suppress the activities of pathogenic bacteria.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Sripathy R. (2019) Phytogenics- Effective modulator of host intervened immune response in poultry production. Engormix.
- Okitoi LO, Ondwasy HO, Siamba DN, Nkurumah D (2007) Traditional herbal preparations for indigenous poultry health management in Western Kenya. Livestock Res Rural Dev 2(1): 1-7.
- Adu OA, Akinboye KA, Akinfemi A (2009) Potency of pawpaw latex as an antihelminthic in poultry production. Bot Res Int 2: 139-142.
- Lin J (2014) Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front Microbiol 5: 33.
- Paul E, Zakaria HM, Ramadhani SON, Namrita L, Antoinette L (2011) Antimycobactrial, antioxidant activity and toxicity of extracts from the roots of Rauvolfia vomitoria. Journal of Medicinal Plants 1(2): 73-80.
- Amole OO, Onabanjo AC, Agbaje EC (1998) Effect of bark extracts of Rauvolfia vomitoria in malaria. Parasitol Int 47: 283-389.
- Khan MR, Omotosho AD (2002) Antibacterial activity of Hygrophila stricta and Peperomia pellucida. Fitoterapia 73(3): 251-254.
- WHO (1998) Quality control methods for medicinal plant materials. A paper presented in Geneva pp: 23.
- Akinyeye RO, Oluwadunsin A, Omoyeni A (2010) Proximate, mineral, anti-nutrients and phytochemical screening and amino acid composition of the leaves of Pterocarpus mildbraedi Harms. Electronic Journal of Environmental Agricultural and Food Chemistry 9: 1322-1333.
- Ajayi IA, Nwozo SO, Adewale A (2010) Antimicrobial activity and phytochemical screening of five selected seeds from Nigeria. International Journal of Biomedical and Pharmaceutical Sciences 4: 104-106.
- AOAC (2000) Association of Official Analytical Chemists. Official Methods of Analysis 19th Edition Washington DC, USA pp: 69-77.
- Harborne JD (1973) Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall London pp: 279.
- Odebiyi A, Sofowora AE (1978) Phytochemical Screening of Nigerian Medicinal Plant Part III. Lloydia 41(3): 234- 246.
- Boham BA, Kocipai AC (1974) Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Pacific Sci 48: 458-463.
- Ngozi E, Ezeomeke, Somadina, Azegba, Promise, et al. (2017) Phytochemical, nutritional and anti-nutritional properties of leaves, stems bark and roots of trees used in popular medicine for the treatment of malaria in South Eastern Nigeria. Journal of Medicinal Plants Research 10(38): 662-668.
- NRC (991) Nutrient Requirement of Domestic Animal, Nutrient Requirement of Rabbits. Second Edition National Academy of Science Washington DC, USA.
- Safwat AM, Sarmiento, Franco, Santos R, Nieves D et al. (2015) Estimating apparent nutrient digestibility of diets containing Leucaena leucocephala or Moringa olifera leaf meals for growing rabbits by two methods. Asian Australian Journal of Animal Science 28(8): 1155-1162.
- Aduku AO, Olukosi JO (1990) Rabbit Management in the Tropics: Production Processing, Utilization, Marketing, Future Prospects. Abuja Nigeria Living Books services, Nigeria.
- Abu Hafsa H, Salem AZM, Hassan AA, Kholif AE, Elghandour MM, et al. (2016) Digestion, growth performance and caecal performance in growing rabbits fed diets containing foliage of browse trees. World Rabbit Sci 16(24): 283-293.
- Unigwe CR, Balogun PA, Okorafor UP, Odah IS, Abonyi FO, et al. (2016) Effect of neem leaf meal on the growth performance and haeamatology of rabbits. World Scientific News 55(16): 51-62.
- Fielding D (1991) Rabbits. The Tropical Agriculturist CTA Macmillan Publishers.
- Niyi A (1997) Prospects of Commercial Rabbit Keeping in Nigeria. Livestock Echo April-June pp. 51-54.
- Aiyesanmi AF, Oguntokun MO (1996) Nutrient composition of Dioclea reflexa seed-an underutilized edible legume. Rivista Italiana delle Sostanze Grasse 73: 521-523.
- Shittu MD, Alagbe JO (2020) Phyto-nutritional profiles of broom weed (Sida acuta) leaf extract. International Journal of Integrated Education 3(11): 119-124.
- Musa B, Alagbe JO, Adegbite Motunrade Betty, Omokore EA (2020) Growth performance, caeca microbial population and immune response of broiler chicks fed aqueous extract of Balanites aegyptiaca and Alchornea cordifolia stem bark mixture. United Journal for Research and Technology 2(2): 13-21.
- Omokore EO, Alagbe JO (2019) Efficacy of dried Phyllantus amarus leaf meal as an herbal feed additive on the growth performance, haematology and serum biochemistry of growing rabbits. International Journal of Academic Research and Development 4(3): 97-104.
- Alagbe JO, Oluwafemi RA (2019) Performance and haematological parameters of broiler chicks gives different levels of dried lemon grass (Cymbopogon citratus) and garlic (Allium sativum) extract. Research in Agricultural and Veterinary Sciences 3(2): 102-111.
- Oluwafemi RA, Omokore EA, Alagbe JO (2020) Effects of dried watermelon and sweet orange peel (DWMOP) meal mixture on the haematological and serum indices of growing rabbits. International Journal of Integrated Education 3(10): 244-250.
- Armand AB, Nicolas YN, Harquin SF, Didier M, Carl MF et al. (2012) Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food and Nutrition Sciences 12(3): 423-432.
- National Research Council (1994) Nutrient requirement for poultry. (9th edn) National Academy Press Washington DC USA.
- Olanipekun MK, Adewuyi D, Adedeji DE (2016) Ethnobotanical importance and phytochemical analyses of some selected medicinal plants in Ado-Ekiti Local Govt. Area. Journal of Herbal Medicine Research 1(3): 7-16.
- Chinelo AE, Ujunwa CN (2017) Investigation of phytochemical and proximate components in different parts of Boerhavia diffusa and B erecta L. Advances in Sciences 2(5): 60-63.
- Alagbe JO, Shittu MD, Eunice Abidemi OjO (2020) Prospect of leaf extracts on the performance and blood profile of monogastric A review. International Journal of Integrated Education 3(7): 122-127.
- Akintayo Balogun Omolere M, Alagbe JO (2020) Probiotics and medicinal plants in poultry nutrition: A review. United International Journal for Research and Technology 2(1): 7-13.
- Labaran I, Lukman OA, Adam JD, Usman M (2016) Analysis of some phytochemicals and minerals found in aqueous stem bark of Albizia lebbeck. Dutse Journal of Pure and Applied 2(1): 231-237.
- Gadde U, Kim WH, Oh ST, Lillehoj HS (2017) Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev 18(1): 26-45.
- Olafadehan OA, Oluwafemi RA, Alagbe JO (2020) Performance, haemato-biochemical parameters of broiler chicks administered Rolfe (Daniellia oliveri) leaf extract as an antibiotic alternative. Advances in Research and Reviews 1: 4.
- Alagbe JO, Oluwafemi RA (2019) Hematology and serum biochemical indices of growing rabbits fed diet supplemented with different levels of Indigofera zollingeriana leaf meal. Progress in Chemical and Biochemical Research 2(4): 170-177.
- Muhammad Amir Maqbool, Muhammad Aslam, Waseem Akbar, Zubair Iqbal (2017) Biological importance of vitamins for human health: A review. J Agric Basic Sci 2(3): 50-58.
- Asensi Fabado MA, Munne´-Bosch S (2010) Vitamins in plants: occurrence,biosynthesis and antioxidant function. Trends Plant Sci 15(10): 582-592.
- Leskova E, Kubikova J, Kovacikova E (2006) Vitamin losses: retention during heattreatment and continual changes expressed by mathematical models. J Food Comp Anal 19: 252-276.
- Alagbe JO, Shittu MD, Bamigboye SO, Oluwatobi AO (2020) Proximate and mineral composition of Pentadiplandra brazzeana stems bark. Electronic Research Journal of Engineering, Computer and Applied Sciences 1(2009): 91-99.
- Keogh JB, Cleanthous X, Wycherley TP, (2012) Increased vitamin B1e intake may berequired to maintain vitamin B1e status during weight loss in patients with type 2diabetes. Diabetes Res Clin Pract 98: 40-42.
- (2017) WH Foods. World’s Healthiest Foods.
- Lanska DJ (2010) Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handbook Clin Neurol 95: 445-476.
- Wong SY, Grant IR, Friedman M, Elliot C, Situ C (2008) Antibacterial activities of naturally occurring compounds against Mycobacterium. Applied Envriron Microbiology 74: 5986-5990.
- Shearer MJ, Newman P (2014) Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res 55(3): 345-362.
- Shearer MJ, Fu X, Booth SL (2012) Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 3(2): 182-195.
- Ogbuewu IP, Okoli IC, iloeje MU (2010) Assessment of blood chemistry, weight gain and linear body measurement of pre-puberal buck rabbits fed different levels of neem (Azadirachta indica) leaf meal. Chilean Journal of Agricultural Research 70(3): 515-520.
- Oluwafemi RA, Egwuiyi GN, Alagbe JO (2020) Effect of feeding Polylathia longifolia leaf meal as partial replacement of wheat offal. European Journal of Agricultural and Rural Education 1(1): 8-16.
- Caspar W (2002) Herbs and botanicals as feed additives in monogastric animals. A paper presented at an 2002 International Symposium on “ Recent advances in Animal Nutrition” held in New Delhi, India.
- El Ghalid OAH, Abd El Hady GM, El Ashry GM, Kholif AE, Olafadehan OA et al. (2019) A newly developed mixture of herbal plants and special extracts and essential oils enhances feed utilization, growth performance and lowers caecal bacteria in rabbits. Indian Journal of Animal Nutrition 36(4): 365.
- Huyghebaert GR, Ducatelle R, Immerseel FV (2011) An update on alternatives to antimicrobial growth promoters for broilers. Vet J 187(2): 182-188.
- Humphrey BD, Klasing KC (2003) Modulation of nutrient metabolism and homeostatis by the immumne system. Proceedings of the European Symposium on Poultry nutrition 60(1): 90-100.
- Toole M, Cooney M (2008) The use of direct fed microbials to mitigate pathogens and enhanced production in cattle. Canadian Journal of Animal Science 9(12): 193-211.
- Saleem R, Ahmed M, Ahmed S I, Azeem M, Khan RA, et al. (2005) Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var.pendula. Phytother Res 19: 881–884.
- Williams RJ, Spencer JP, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Rad Biol Med 36(7): 838-849.
- Alagbe JO (2017) Effect of dietary inclusion of Polyalthia longifolia leaf meal as phytobiotic compared with antibiotics on performance, carcass characteristics and haematology of broiler chicken. Scholarly Journal of Agricultural Science 7(3): 68-74.
- Adisa RM, Choudhary EA, Adenoye GA, Olorunsogo OO (2010) Hypoglycaemic and biochemical properties of Cnestis ferruginea. Afr J Tradit Complement Altern Med 7(3): 185-194.
- Kasolo JN, Gabriel S, Bimenya LO, Joseph O, Ogwal-Okeng JW (2010) Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res 4(9): 753-757.
- Hassan HS, Sule MI, Musa AM, Musa KY, Abubakar M S et al. (2012) Anti-inflammatory activities of Crude Saponin Extracts from Five Nigerian Medicinal Plants. Afr J Tradit Complement Altern Med 9(2): 250-255.
- Hollman PC (2001) Evidence for health benefits of plant phenols: Local or systemic effects? J Sci Food Agric 81: 842-852.
- Khan RU, Naz S, Nikousefat Z, Tufarelli V, Laudadio V (2012) Alternative to antibiotics in poultry feed. World's Poult Sci J 68: 401-408.
- Kamel C (2001) Natural Plant Extracts: Classical Remedies Bring Modern Animal Production Solutions. In Sow Feed Manufacturing in the Mediterranean Region Improving safety From Feed to Food, Brufau J (Ed.) CIHEAM Reus Spain 54: 31-38.
- Ishihara N, Chu DC, Akachi S, Juneja LR (2001) Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest Prod Sci 68: 217-229.
- Alagbe JO (2019) Proximate, mineral and phytochemical analysis of Piliostigma thonningii stems bark and roots. International Journal of Biological Physical and Chemical Studies 1(1): 1-7.
- Alagbe JO (2020) Proximate phytochemical and vitamin compositions of Prosopis africana stem bark. European Journal of Agricultural and Rural Education1(4): 1-7.
- Ereifej K, Ranya E, Taha R, Ali AM, Muhammad HA (2012) Minerals proximate composition and their correlations of medicinal plants from Jordan. Journal of Medicinal Plant Research 6(47): 5757-5762.
- Alagbe JO (2020) Effect of dietary supplementation of Cymbopogon Citratus oil on The Performance and Carcass characteristics of broiler chicks. European Journal of Biotechnology and Bioscience 8(4): 39-45.
- Olafadehan OA, Oluwafemi RA, Alagbe JO (2020) Carcass quality, nutrient retention and caeca microbial population of broiler chicks administered Rolfe (Daniellia oliveri) leaf extract as an antibiotic alternative. Journal of Drug Discovery 14(33): 146-154.
- Youdim KA, Shukitt-Hale B, MacKinnon S (2000) Polyphenolics enhance red bloodcell resistance to oxidative stress: in vitro and in vivo. Biochim Biophys Acta 1523(1): 117-122.
- Wagner CL, Greer FR (2008) Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122: 1142-1152.
- Olajumoke OO, Soretiwa SA, Lawrence OO (2002) Phytochemical screening, anti-nutrient composition, proximate analysis and the antimicrobial activities of the aqueous and organic extracts of bark of Rauvolfia vomitoria and leaves of Peperomia pellucida. International Research Journal of Biochemistry and Bioinformatics 2(6): 127-134.
- Abubakar M, Ibrahim U, Yusuf AU, Mohammad AS, Adamu N (2014) Growth performance, carcass and organ characteristics of growing rabbits fed graded levels of Moringa olifera leaf meal in diets. Bayero Journal of Pure and Applied Sciences 8(2): 7-9.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...