Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Carbon Stock of Tree Species and its Biodiversity in Community Forests of Dang, Nepal Volume 4 - Issue 1
Anju Subedi1, Dipika Badal1, Ram Asheshwar Mandal2* and Bijay Kumar Yadab3
- 1Kathmandu Forestry College, Kathmandu, Nepal
- 2School of Environmental Science and Management (SchEMS), Kathmandu, Nepal
- 3Institute of Forestry, Hetauda Tribhuvan University Nepal
Received: August 28, 2020; Published: September 09, 2020
Corresponding author: Ram Asheshwar Mandal, School of Environmental Science and Management (SchEMS), Kathmandu, Nepal
DOI: 10.32474/CDVS.2020.04.000179
Abstract
Carbon stock and biodiversity have an intricate relationship. Despite the important relationship between these components the studies related to it are very limited. Thus, this research was objectively conducted to estimate species wise carbon stock, soil organic carbon and biodiversity. Three community forests namely Sungure, Goraksha and Baghkhor of Dang district were selected for the study. Total 45 nested sample plots having 12.61m, 5.64m, 2.82m and 1.87m radius for tree, pole, sapling and seedling respectively were established to collect the data. Additionally, 45 soil samples were collected from 0-10, 10-20 and 20-30 cm depth from centre of the plot. The diameter and height and tree species were recorded. The biomass of each species was calculated using equation Chave [1] and the value was converted into carbon. Soil carbon was analyzed using Wakley and Black method. The biodiversity was estimated using Shannon Weinner and Simpson’s Diversity Index. Shorea robusta was the dominant species in the community forests, thus the carbon stock was the highest around (mean±SE) 33.23±0.23, 28.15±0.25 and 20.61±0.65 ton/ ha in Baghkhor, Goraksha and Sungure community forests respectively. The carbon stock of Shorea robusta was 38.61 to 41.31% which can suppress other species. One-way ANOVA and Tukey’s b test showed that the mean carbon stock of Shorea robusta in the community forests was significantly differed at 95% confidence level. The highest contribution of carbon stock was of soil around 64.77 (57.52%) ton/ha in Baghkhor community forest. The soil carbon stock of Baghkhor community forest was 26.65, 21.55, 16.57 ton/ha at 0-10 cm, 10-20 cm, 20-30 cm respectively. The species richness was the highest 17 in Sungure community forest. The Simpson index was 0.32, 0.34 and 0.34 in Sungure, Goraksha and Baghkhor community forests respectively while Shannon- Weiner diversity index values were 1.31, 1.13 and 1.3 in the community forests respectively. The research will be useful to understand the carbon stock and biodiversity in community forest.
Keywords: Carbon, volume, biodiversity, importance value index
Introduction
The carbon sequestration has been playing a vital role to
mitigate and adapt against the climate change. In fact, the forest
is sink and source of carbon as well. On the other hand, the forest
is the source of biodiversity too. So, conserving the forest means
conserving the forest carbon as well as biodiversity. However, the
inverse process is also true. About 20 % greenhouse gases can be
cut conserving the forest [2,3]. Importantly, conserving the forest is
able to address the issues of climate change as well as biodiversity.
The united national framework convention on climate change and
convention on biodiversity highly emphasize on the conservation
of the forest [4-6].
Carbon sequestration is the process of removing additional
carbon from the atmosphere and depositing it in other reservoir
principally through change in land use [7]. Mainly, two mechanisms
have been working to incentivize to credit the carbon stock in
the forest [8]. The Reducing Emission from Deforestation and
Degradation (REDD+) and Clean Development Mechanism (CDM)
[9]. The REDD+ mechanism also value the biodiversity but considers this as the co-benefit, though biodiversity is equally
important in the world [10,11]. Thus forest conservation is one of
important element of REDD+ mechanism. Developing countries are
assumed as foundation of REDD+ mechanism and implementation
of convention of biological diversity.
Developing countries are committed to work under the REDD+
mechanism and to work for convention on biological diversity [12].
In fact over 80 countries have been working under the forest carbon
partnership facility under the REDD+ mechanism [13,14]. Besides,
total 196 countries are the member of convention on biological
diversity [15,16]. Both mechanisms need to build the database
and update regularly. Community managed forests functioning
to upscale the carbon sequestration as well as the biodiversity.
In this context, parties need the reliable information regarding
forest carbon and biodiversity. This can be done through intensive
studies. Community forests are famous management approach in
the world to assure the increasing carbon stock but the biodiversity
conservation is questionable. The examples of community forest
management for carbon sequestration and biodiversity of Cost
Arica, Papua New Guinea, Mexico, and India including Nepal are
evidence.
Community forests, collaborative forest, leasehold forest,
buffer zone forest are decent ground of carbon sequestration and
biodiversity conservation in Nepal [17,18]. There are over 23,000
community forests, more than 70 buffer zone community forests,
23 collaborative forests in Nepal. All these forests are potential
for forest carbon credit. However, biodiversity conservation in the
community forest especially where the management practices are
intensively applied is in doubt. This research tried to evaluate the
carbon stock of tree species in the community forests, soil carbon
and biodiversity as well.
Materials and Methods
Study area
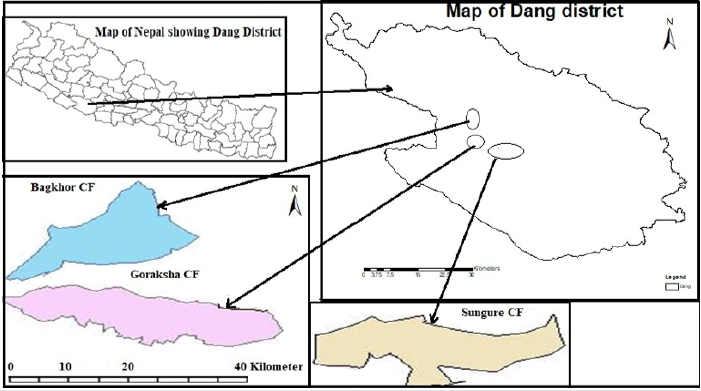
Three community forests namely Sungure, Goraksha and Baghkhor community forest of Dang district, Nepal were selected as the study area. Dang is the largest valley of Nepal and Asia. It lies in Province no. 5. The forest area of this district is 192,155 ha around 65% of total land area. It ranges between 28°30’.00’ʹ N and 82°18’.00’ʹ E. Sungure community forest lies in Ghorahi sub metropolitan 18, covering 84.64 ha. Goraksha community forest lies in Ghorahi sub metropolitan city 18, Dabari, covering 81.89 ha and Baghkhor community forest lies in Ghorahi sub metropolitan city 16, Bahundadha, covering 82.91 ha. The Shorea robusta, Terminalia tomentosa, Dalbergia sisso and Acacia catechu are the most common tree species in the community forests (Figure 1).
Sampling design
Stratified random sampling was applied based on the stage of the tree to collect the data. The forest areas was surveyed and mapped. Next, total 45 sample plots 15 plots in each community forest were distributed randomly on the map. Total nested sample plots of 12.61m, 5.64m, 2.82m and 1.87m radius for tree, pole, sapling and seedling respectively were established in the field to collect the data [19].
Data collection
The diameter at breast height, height and tree species were recorded. Additionally, 45 soil samples were collected from 0-10, 10-20 and 20-30cm depth from the center. Then sapling (5cm< dbh >1cm), seedlings were counted and fresh weights were noted.
Data Analysis
All collected data were analyzed quantitatively. The biomass
was calculated using equation of Chave [1]. AGTB = 0.0509* ρ D2H,
where AGTB = Above-Ground Tree Biomass (kg); ρ = wood specific
gravity (kgm-3); D = tree diameter at breast height (cm) and H =tree
height (m). The Above Ground Sampling Biomass (AGSB) (kg) was
calculated using formula; Ln (AGSP) = a +b ln (DBH); Ln is natural
log, a & b are constants. D is the diameter at breast height (cm)
[20,21]. The Below Ground Biomass (BGB) was calculated using
root shoot conversion factor 0.125 [22]. Besides, the fresh weight
was taken of regeneration (sapling and seedling) and dried in
the lab to find the dry weight. The unitary method was applied to
determine the biomass of regeneration. The carbon stock density
of soil organic carbon was calculated using Walkley and Black [23]
method.
SOC = ρ × d × %C, where SOC = soil organic carbon stock per
unit area [t/ ha], ρ (Bulk Density g/cc) = (oven dry weight of soil)/
(volume of soil in the core), d = the total depth at which the sample
was taken [cm], and %C = carbon concentration [%].
Finally the carbon stock was calculated where conversion factor
used was 0.47 on biomass of total pools except soil carbon.
Total carbon = C (AGTB) + C (AGSB) + C (BGB) + C (LHG) + SOC
Where, C (AGTB) = carbon in aboveground tree biomass [Mg C
/ha]; AGTB*0.47
C (AGSB) = carbon in aboveground sapling biomass [Mg /ha];
AGSB * 0.47
C (BGB) = carbon in below ground biomass [Mg C /ha]; BGB *
0.47
C (LHG) = carbon in litter, herbs, and grasses [Mg C/ha];
LHG*0.47
SOC = soil organic carbon [Mg C/ha]
For calculation of Biodiversity Index, the species diversity of
the forest tree community was calculated using different indices as
mentioned below:
Simpson’s diversity index (D) =i2, where pi is the total
individuals in a species community,
Shannon-Weiner Biodiversity Index (H`) = -ΣPi*Log Pi, where
Pi = ni / N, where, N=Total no of species, ni= no. of individuals of
species and Pi= ni/N
Statistical analysis
Normality test was done to check the normality of the data set. The data were normal thus one way ANOVA test and Tukey’s b test was applied to evaluate whether soil carbon was significantly differed among the community forests.
Result
Species wise carbon stock in community forests
The carbon stock was varied according to species in the community forests. Shorea robusta was the dominant species in the community forests, thus the carbon stock was the highest around (mean±SE) 33.23±0.23, 28.15±0.25 and 20.61±0.65 ton/ha in Baghkhor, Goraksha and Sungure community forests respectively. The estimated carbon stock was followed by Terminalia alata with 10.76±0.37, 10.32±0.34 and 6.93±0.3 in Baghkhor, Goraksha and Sungure community forests respectively. The proportion of carbon stock of Shorea robusta was 38.61 to 41.31% which can suppress other species to regenerate and growth as this is a valuable timber species, users have no interest to promote other species in the community forest (Table 1). One-way ANOVA and Tukey’s b test showed that the mean carbon stock of carbon of Shorea robusta in these community forests at 95% confidence level since the p-value was less than 0.05. Similar condition was found of carbon stock of Terminalia alata but Tukey’s be showed that the mean carbon stock was of Goraksha and Baghkhor community forest was significantly differed from Sungure community forest at 95% confidence level.
Table 1:Species wise carbon stocks.

Note: The word in parenthesis is local name of the tree species.
Proportion of carbon stock based on the stage of the tree
The highest contribution of carbon stock was of soil around 64.77 (57.52%) in Baghkhor community forest. Maximum soil carbon was 50.40 % in Sungure community forest. The contribution of tree carbon stock ranges from 23.24, 33.06 and 34.27 ton/ha which were around 26.01, 26.87 and 25.14% of total carbon in the community forests (Table 2). The soil carbon stock was also compared based on the depth layers in each community forest. It was found that the soil carbon stock of Baghkhor community forest was found to be highest in three layers (0-10 cm, 10-20 cm, 20- 30 cm) with values, 26.65, 21.55, 16.57 ton/ha respectively than that of other community forest i.e. Goraksha community forest having value 26.30, 17.38, 14.57 ton/ha and Sungure community forest having value 18.97, 14.20, 11.85ton/ha in three layers (0-10 cm, 10-20 cm, 20-30 cm) respectively (Table 3). One way ANOVA showed that, there was a significant difference in soil carbon in different community forest and similar result was found applying the Tukey’s b test at 95% confidence level.
Biodiversity Indices in community forests
Species richness: The species richness was the highest 17 in Sungure community forest which was 15 in Goraksha community forest and only 12 tree species in Baghkhor community forest. Total 17 plant species were found in Sungure community forest, out of which about 13 plant species were common in all three selected forests. Shorea robusta, Melaleuca leptospermum (Maluku), Dalbargia sissoo, Terminalia alata , Garuga pinnata (dabdabe), Eugelharlia spicata (Mahuwa), Mallotus philippensis (Rohini), Syzygium cumini (Jamun), Aesandra butyracea (Churie), Anthocephalus chinensis (Kadam), Trichilia connoaroides (Aakha tarya), Phyllanthus emblica (Amala), Pyracantha crenulata (Dhayro), Pterocarpus santalinus (Kattai), Rhus wallichii (Bhalayo), Acacia catechu (khair) and Dillenia pentagyna (Tantari).
Simpson’s diversity index value and Shannon- Weiner diversity index: The Simpson index was 0.32, 0.34 and 0.34 in Sungure, Goraksha and Baghkhor community forests respectively while the Shannon- Weiner diversity index values were 1.31, 1.13 and 1.3 in the community forests respectively (Table 4). Statistically, ANOVA test also showed that there was no difference in values of Simpson’s index and Shannon-Wiener index among three community forests at 5% level of significance.
Discussion
The study of total carbon stocks in three selected community forest found that the estimated total per ha biomass carbon of Sungure, Goraksha and Baghkhor community forest was 44.32, 64.78 and 71.52ton respectively. Based on comparison of species in the community forest it was found that, the estimated carbon stock of Shorea robusta was highest, having value of 33.23±0.23 ton/ha in Baghkhor community forest, 28.15±0.25 ton/ha in Goraksha community forest and 20.61±0.65 ton/ha in Sungure community forest. Mandal [24] showed that the carbon stock of Shorea robusta was nearly 52.31ton/ha, which is slightly higher than the present study. The main reason of highest carbon stock of Shorea robusta was because of the dominancy of this species in the community forests.
Based on report published by department of research and survey, estimated tree carbon (>10cm) was 104.47 ton/ha which is slightly higher than present study as the present study. This may be due to the potential of forest to sequester carbon depends on the forest type, age of forest and size of trees [25-27]. The density of carbon stock values would vary according to the geographical location, plant species, age of the stand, aboveground input received from leaf litter, decomposition of fine roots below ground, management practices and other operating ecological factors [28- 30].
Khanal [31] estimated that the above ground tree carbon was 40.02 ton/ha and the above ground tree carbon of Sungure, Goraksha and Baghkhor community forest is found to be 44.32, 64.78 and 71.52 ton/ha which is slightly higher than the report presented by Khanal [31]. Similarly, the study done by KC showed that the value of above ground carbon of Shorea robusta in Jhilbang community forest of Pyuthan was 82.54 ton/ha which is slightly higher than the present study. The above ground carbon variation in age of the stand ranged from 18-75 years, variation in carbon per hectare from 34.70-97.86 ton/ ha [32]. Moreover, Shrestha [17] estimated carbon stocks in trees showed that 219 ± 34 Mg ha–1. Shrestha assessed the net above ground carbon stock in six community forests of the Dolakha district, Nepal where it was found that the community forests accumulates approximately 117.44 tons of carbon in total.
Based on the report of department of forest research and
survey (DFRS), the soil organic carbon of Terai was estimated as
33.66 ton/ha and the soil organic carbon found from the present
study is 64.77, 58.26 and 45.04 ton/ha which is slightly higher
than the DFRS report. Ghimire [33] reported that the soil organic
carbon of Makawanpur district is 58.82 ton/ha. Mandal [34]
reported soil organic carbon in Chyandanda community forest
of Mahottari district is 58.22 ton/ha which is slightly higher
than Sungure community forest having value and lower than
Baghkhor community forest but approximately similar to Goraksha
community forest. The study done by Pandey and Bhusal [35]
showed that soil organic carbon of Terai community forest was 95.1
ton/ha which is higher than the present study.
The soil organic carbon of this study shows that the carbon
content decreased with the increase in soil depth in three forest
areas. The total soil carbon stock in depth 0-10 cm contained greater
value than in depth 10-20 cm and followed by 20-30cm respectively.
This may be because of the higher aggregate of humus in the top
layer of the soil profile in the forest blocks, decreased content of
organic matter with the increased level of soil depth and also due
to soil organic carbon diminishing with the depth regardless of
vegetation, soil texture and clay size fraction [36,37]. The decrease
in soil carbon with the increase in soil depth was supported by the
following studies. The study conducted by Sharma [38] in Shree
Salumbudevi community forest of Pukhulachhi VDC, Kathmandu,
which showed the maximum amount of the soil organic carbon was
obtained in the upper layer 0-10 cm as compared with the lower
layer 20-30 cm. The comparative reports and the present study thus
shows that the amount of soil carbon decreases with increasing soil
depth in the studied community forest.
The Simpson’s and Shannon’s indices of Sungure, Goraksha
and Baghkhor community forest were found to be 0.32, 0.34, 0.34
and 1.31, 1.13, 1.3 respectively. Lower the Simpson’s index value
higher the diversity and higher the Shannon’s index value higher
the diversity. The biodiversity indices values were slightly differed
between the forests. The study done by Mandal, showed that the
Shannon-Wiener Biodiversity Index was the highest 2.33 in Banke-
Maraha collaborative forest (CFM), and it was the lowest 2.21 in
Gadhanta-Bardibas CFM. Similarly, the Simpson index values
were 0.39, 0.41 and 0.44 in Banke-Maraha. Tuteshwarnath and
Gadhanta-Bardibas CFM respectively, this was not similar to the
value of community forest because of the application of silviculture
operation done in our study areas. The silviculture operation
reduce the biodiversity [39-70].
Conclusion and Recommendation
The dominant species was Shorea robusta in the community forest, thus the carbon stock was the highest of this species. The carbon stock contribution was about similar proportion of biomass and soil. The soil carbon decreased with the increased soil depth. Simpson’s diversity index and Shannon-Wiener index values showed that, the plant species diversity was not significantly different in three community forests. The study also recommended that the assessment of biomass carbon stocks and biodiversity of community forest should be given priority to include in operational plan of community forest.
References
- Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, et al. (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145: 87–99.
- IPCC (2000) The Intergovernmental Panel on Climate Change, Special Report on Land Use, Land-Use Change and Forestry. Cambridge University Press, Cambridge, UK.
- Hepburn C, Stern N (2008) A new global deal on climate change. Oxford Review of Economic Policy 24(2): 259-279.
- Gautam KR (2002) Carbon Sequestration in agro forestry and annual cropping system in Inner Terai, Central Nepal. M Sc. thesis submitted to Agriculture University of Norway, Norway.
- Williams M (2003) Deforesting the Earth – from Prehistory to Global Crisis. The University of Chicago Press, Chicago, USA.
- Acharya KP, Dangi RB, Tripathi DM, Bushley BR, Bhandary RR, et al. (2009) Ready for REDD? Taking Stock of Experiences, Opportunities and Challenges in Nepal. Kathmandu, Nepal: ICIMOD (International Centre for Integrated Mountain Development).
- Pan Y, Birdsey R, Hom J, McCullough K (2009) Separating effects of changes in atmospheric composition, climate and land-use on carbon sequestration of US Mid-Atlantic temperate forests. Forest Ecology and Management259(2): 151-164.
- Lederer M (2011) From CDM to REDD+What do we know for setting up effective and legitimate carbon governance?. Ecological economics 70(11): 1900-1907.
- Angelsen Arild, Charlotte Streck, Leo Peskett, Jessica Brown, Cecilia Luttrell (2008) What is the right scale for REDD." In Moving ahead with REDD: issues, options and implications, 3: 31-40.
- Brown D, Seymour F, Peskett L (2008) How do we achieve REDD co-benefits and avoid doing harm. Moving ahead with REDD: issues, options and implications, pp. 107-118.
- O’Connor D (2008) Governing the global commons: Linking carbon sequestration and biodiversity conservation in tropical forests. Global Environmental Change18(3): 368-374.
- Corson C, MacDonald KI (2012) Enclosing the global commons: the convention on biological diversity and green grabbing. Journal of Peasant Studies 39(2): 263-283.
- Hernandez-Gongora L (2020) REDD plus governance needs a driver and more fuel. The case of Quintana Roo, Mexico(Doctoral dissertation).
- Miles WB (2020) The invisible commodity: Local experiences with forest carbon offsetting in Indonesia. Environment and Planning E: Nature and Space, 2514848620905235.
- McPherson M (2020) China’s Role in Promoting Transboundary Resource Management in the Greater Mekong Basin (GMB).
- Woldegiorgis B (2020) A history and policy analysis of Forest Governance in Ethiopia and REDD+.
- Shrestha BM, Singh BR, Sitaula BK, Lal R, Bajracharya RM (2007) Soil aggregate and partial associated organic carbon under different land use in Nepal. Soil Science Society of America Journal 71(4): 1194-1203.
- Shrestha BP (2008) Carbon Sequestration in Schima Castonopsis Forest. A case study from Palpa District. The Greenery 7: 34-40.
- HMG (2002) Nepal Biodiversity Strategy, government strategy paper, His Majesty’s Government of Nepal/Ministry of Forest and Soil Conservation, Kathmandu, Nepal.
- IPCC (2006) Good Practice Guidelines for National Greenhouse Gas Inventories. Switzerland: Intergovernmental panel on climate change.
- MFSC (2011) Forest carbon measurement guidelines, Climate Change and REDD Cell, Ministry of Forestry and Soil Conservation, Kathmandu, Nepal.
- MacDicken KG (1997) A Guide to Monitoring Carbon Storage in Forestry and Agro forestry Projects. Forest Carbon Monitoring Programme, Winrock International Institute for Agricultural Development, Littlerock, Arkansas, USA.
- Walkey AE, Black JA (2002) “An examination of the method for Determining soil organic method & proposed modification of the chromic acid Titration method”.
- Mandal RA, Jha PK, Dutta IC, Thapa U, Karmacharya SB (2016) Carbon sequestration in tropical and subtropical plant species in collaborative and community forests of Nepal. Advances in Ecology.
- Magar KB (2009) Carbon Stock in Community Managed Shorea Robusta Forests of Dhading District, Nepal. Master of Science in Botany (Plant Ecology), Tribhuvan University, Nepal.
- Nair PKR (2012) Carbon sequestration studies in agroforestry systems: a reality-check. Agroforestry Systems86(2): 243-253.
- Udawatta RP, Jose S (2012) Agroforestry strategies to sequester carbon in temperate North America. Agroforestry Systems 86(2): 225-242.
- Kurz WA, Dymond CC, White TM, Stinson G, Shaw CH, et al. (2009) CBM-CFS3: a model of carbon-dynamics in forestry and land-use change implementing IPCC standards. Ecological modelling 220(4): 480-504.
- Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press, UK.
- Nautiyal N, Singh V (2013) Carbon Stock Potential of Oak and Pine Forests in Garhwal Region in Indian Central Himalayas. Journal of Pharmacognosy and Phytochemistry 2: 43-48.
- Khanal Y (2008) Valuation of carbon sequestration and water supply service in community forest of Palpa district, Nepal.
- Baral SK., Malla R, Ranabhat S (2009) Above-ground carbon stock assessment in different forest types of Nepal. Banko Janakari 19(2): 10-14.
- Ghimire P, Kafle G, Bhatta B (2018) Carbon stocks in Shorea robusta and pinus roxburghii forests in Makawanpur District of Nepal. Journal of Agriculture and Forestry University 2: 241-248.
- Mandal R, Dutta IC, Jha PK, Karmacharya S (2013) Relationship between Carbon Stock and Plant Biodiversity in Collaborative Forests in Terai, Nepal.
- Pandey HP, Bhusal M (2016) A comparative study on carbon stock in Sal (Shorea robusta) forest in two different ecological regions of Nepal. Banko Janakari 26(1): 24-31.
- Trujilo W, Amezquita E, Fisher MJ, Lal R (1997) Soil organic carbon dynamics and landuse in the Colombian Savannas I. Aggregate size distribution. In: Lal R, Kimble JM, Follett RF (Eds.), Soil Processes and the Carbon Cycle pp. 267-280.
- Cambi M, Certini G, Neri F, Marchi E (2015) The impact of heavy traffic on forest soils: A review. Forest ecology and management338: 124-138.
- Sharma K (2011) An Assessment of Carbon Sequestration Potential of Community Managed Forest with reference to Shree Salumbudevi Community Forest, Pukhulachhi VDC-9 Kathmandu, M.Sc. Dissertation, College of Applied Sciences, Kathmandu, Nepal.
- Acharya KP (2004) Does Community Forests Management supports biodiversity conservation? Evidences from two community forests from the mid hills of Nepal. J. For. Livelihood 4: 44-54.
- Torras O, Saura S (2008) Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean. Forest Ecology and Management 255(8-9): 3322-3330.
- Ciancio O, Nocentini S (2011) Biodiversity conservation and systemic silviculture: concepts and applications. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology145(2): 411-418.
- Bajracharya RM, Lal R, Kimble JM (1998) Soil organic carbon distribution in aggregates and primary particle fractions as influenced by erosion phases and landscape position. In Soil Processes and the CarbonCycle (Eds) Lal R., Kimble J., Follett. R and Stewart B.A. CRC Press, Boca Raton, Florida, USA, pp. 353–367.
- Bass Stephedn, Dubios Olivier, Mouro Costa, Pedro, Pinard, Michelle Tipper, et al. (2000) Rural Livelihoods and Carbon management. IIED natural resource issues paper.
- Brown S, Sathye J, Cannel M, Kauppi PE (1996) Mitigation of carbon emissions to the atmosphere by forest management. Commonwealth forestry Review 75(1): 80-91.
- Byrne KA, Milne R (2006) Carbon stocks and sequestration in plantation forest in the Republic of Ireland. Forestry 79(4): 361-369.
- Dahal N (2004) Final report Kyoto: Think Globally, Act Locally (KTGAL).King Mahendra Trust for Nature Conservation, Lalitpur, Nepal.
- Davidson EA, Trumbore SE, Amundson R (2000) Biogeochemistry: soil warming and organic carbon content. Nature 408(6814): 789.
- DOF (2003) Community Forestry Inventory Guideline, Community Forests Division, Department of Forests, Kathmandu, Nepal, p. 10-20.
- Dhital A (2009) Reducing Emissions from Deforestation and Forest Degradation (REDD) in Nepal: Exploring the Possibilities. Journal of Forest and Livelihood 8: 1.
- FAO (2006) Global Forest Resources Assessment 2005. In Progress towards Sustainable Forest Management. FAO, Rome.
- FAO (2010) ''Global Forest Resources Assessment'', Main report .FAO forestry paper. 163: 10-15.
- IPCC (2001) Climate Change 2001. Cambridge University Press, Cambridge, UK.
- IPCC (2007) "Climate Change 2007: Synthesis Report", Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team, R. K. Pachauri, A. Resinger, IPCC, Geneva, Switzerland, pp. 104.
- Jodha NS (1990) Rural Common Property Resources: Contributions and Crisis. Economic and political Weekly, 21: A65-A78.
- Dipak, KC (2011) An assessment of carbon sequestration in hill Sal forest. IOF Hetauda.
- Khayamali, BP (2010) Assessing carbon benefits from Neelbarahi and Gauradev community forest of Bhakatapur.M.Sc. Dissertation, Department of Enviromental Science, Khwopa college Bhakatapur, Nepal.
- Khun V, Sasaki N (2014) Re-Assessment of Forest Carbon Balance in Southeast Asia: Policy Implications for REDD+. Low Carbon Economy 5: 153-171.
- Majumdar K, Choudhary BK, Datta BK (2016) Above ground woody biomass, carbon stocks potential in selected tropical forest patches of Tripura, Northeast India. Open Journal of Ecology 6(10): 598.
- MFSC (2000) “National Biodiversity Action Plan.” Ministry of Forest and Soil Conservation, HMG of Nepal, Global Environment Form, UNDP, p.11-30.
- MFSC (2010) Forest Carbon Measurement Guidelines. Ministry of Forest and Soil Conservation, Kathmandu, Nepal.
- Negi JDS, Manhas RK, Chauhan PS (2003) Carbon allocation in different components of some tree species of India: A new approach for carbon estimation in Shrestha, B.P. Carbon Stocks in the Oka and Pine forests in Salyan District, Nepal. Banko Jankari 23(2): 30.
- Pearson TR, Brown SL, Birdsey RA (2007) Measurement guidelines for the sequestration of forest carbon. Northern Research Station, Department of Agriculture, USA.
- Phat N, Knorr W, Kim S (2004) Appropriate Measures for Conservation of Terrestrial Carbon Stocks Analysis of Trends of Forest Management in Southeast Asia.
- Post WM, Peng TH, Emanuel WR, King AW, Dale VH, et al. (1990) The global carbon cycle. Am Sci 78: 310–326.
- Ross WG, Sheikh PA (2010) “Deforestation and Climate Change,” Congressional Research Service.
- Sapkota IP, Tigabu M, Oden PC (2010) “Tree diversity and regeneration of community managed Bhabar lowland & Hill Sal forests in central regional of Nepal”.
- Sharma DB, Karky SB, Dahal N, Chapagain N, Basnet B (2004) Prospects and Challenges in Bringing Nepal’s Community Forestry under Kyoto Protocol’s Carbon Trading Regime In. In Twenty-five Years of Community Forestry Proceedings of the Fourth National Workshop on Community Forestry p. 4-6.
- Singh SP, Sah PP, Tyagi V, Jina BS (2005) Species diversity contributes to productivity-evidence from natural grassland communities of the Himalaya.” Current Science 89(3): 548–552.
- Stern N (2006) The Economics of Climate Change: The Stern Review. Cambridge, Cambridge University press, UK.
- Thapa P (2014) Comparison of Carbon Stocks in Community Forests of Lamahi Corridor, Kailali in Terai and Basanta Corridor, Dang in Inner Terai.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...