Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
Blood Testosterone Level Affects Sex Ratio of Bull Semen Volume 4 - Issue 1
Muna Kholghi2, Jalal Rostamzadeh2, Mohammad Razmkabir2 and Farid Heidari1*
- 1Department of Animal Biotechnology, Faculty of Agriculture Biotechnology, National Institute of Genetic Engineering and Biotechnology, Iran
- 2Department of Animal Science, University of Kurdistan, Sanandaj, Iran
Received: August 03, 2020; Published: August 18, 2020
Corresponding author: FaridHeidari, Department of Animal Biotechnology, Institute of Agricultural Biotechnology, National Institute of Genetic Engineering and BiotechnologyShahrak-e Pajoohesh, km 15 Tehran - Karaj Highway, Tehran, Iran
DOI: 10.32474/CDVS.2020.04.000177
Abstract
Sex ratio has a direct impact on livestock economy and controling sex-linked genetically diseases. Offspring sex ratio is affected by such various factors. One of these factors is the Y/X-hromosome bearing sperm ratio in fertile specimen. This study was conducted to explore the effect of testosterone concentrations of blood and semen on the relative frequency of Y/X -chromosome bearing sperm in Holstein bovine semen. Blood and semen testosterone level were measured by ELISA technique. Quantitative real-time PCR was performed to estimate the ratio between sperm with Proteolipid Protein (PLP) and the Sex-Related Y (SRY) genes, locating on non-homologous regions of X and Y chromosomes. Blood and semen samples of 26 Holstein bovines were taken simultaneously. DNA was extracted from semen sampls and real-time PCR was performed to amplify the fragments of 90, 89, and 79 base pairs (bp) for PLP, SRY and PAR (as reference) genes, respectively. Wide variation was shown in Y- and X - chromosome bearing sperm, ranging between 18-82%. The least mean square of Y-bearing sperm (1.23±0.15) was significantly higher than that of X-bearing sperm (0.71±0.02). The correlation coefficients of SRY and PLP with blood and semen concentration of testosterone were 0.38, 0.47, -0.67 and -0.60, respectively. The results demonstrated that higher testosterone levels are probably associated with a higher proportion of Y- bearing sperm. A significant positive correlation (P<0.05) was detected between the age of cattle and the ratio of Y-bearing sperm. The testosterone concentration of blood and semen was positively correlated to the cattle age (P<0.05). The results may provide insights into the effects of paternal testosterone on sex ratio of sperm transferred to females.
Keywords: Real-time PCR, Semen, Sex ratio, Testosterone
Introduction
Introduction
Offspring sex ratio is an important statistic index defined as the proportion of males to females newborn[1], which expected to be 1:1 in populations. But recent researches have shown this proportion can vary significantly from the expected rate [2,3]. In the livestock industry, sex preselection of offspring has a special economic importance, therefore causes and mechanisms of sex selection are hot subjects for investigators[4-6]. From 1970 onwards, factors affecting sex ratio have been studied and role of breed and genetic, season, nutrition, age and weight of parent, gestation periods, male ejaculation frequency, time of insemination, different movement speed of the X- and Y-chromosome bearing sperm were studied[7-10]. These factors may be affect cervical mucus, metabolites and female reproductive tract secretion and vaginal pH [11]. Ability of father to bias offspring sex ratio has been dismissed given the expectation of an equal proportion of Y/Xchromosome bearing sperm during ejaculation. This expectation has been recently refuted[12]. Gomendio[13] reported a strongly sexually dimorphic species and a classic example for large variance across males in reproductive success-to show that fathers can bias sex ratio at birth. More fertile fathers produce more sons and less fertile produce more daughters. Saragusty [14] shown that variation in the ratio of Y/ X-chromosome bearing sperm in the ejaculation associates with variation in the sex of the offspring produced. Analysis of large dataset was shown males with higher reproductive success have a higher proportion of male offspring, and also such sex ratio bias is adaptive[15].
It has been reported that the concentration of testosterone
plays a key role in offspring sex ratios in different mammals, as
there was a positive correlation between high concentration of
testosterone and bias in sex ratio of offspring toward males[16,17].
Recent advances in molecular genetics has been resulted in the
development of a variety of techniques (such as real-time PCR,
fluorescent in-situ hybridization (FISH), and flow cytometry)
for accurate estimation of X- and Y-chromosome bearing sperm.
Among these techniques, real-time PCR provides an easy-to-use
context for estimation of the copy number of genes [18]. The
sex-related Y gene (SRY), which is located on the short arm of Y
chromosome close to the centromere, has been frequently used as a
marker for detection of Y-chromosome bearing sperm. SRY involves
in initiation of transcription, processing of mRNA, participation in
spermatogenesis, motility of sperms, interaction between sperm
and ovum, and testosterone production[19-21]. SRY can affect
the viability of Y chromosome and its role in population ratio of
X and Y chromosome-bearing sperm suggested to examined[22].
The proteolipid protein (PLP) gene, on the other hand, is routinely
used for detection of X-chromosome bearing sperm. PLP locates
on non-homologous region of X chromosome and is expressed in
all nervous and non-nervous tissues. The increasing expression of
PLP under different physiological conditions has been shown to
stimulate apoptosis process [23].
Despite the high frequency of studies on the role of testosterone
on offspring sex ratio in different mammals, the potential effect
of testosterone concentration of bovine in the ratio of his Y/Xchromosome-
bearing sperm has been poorly studied. In this
research, The effect of blood and semen testosterone concentration
on the viability and proportion of Y/X -chromosome bearing sperm
in male Holstein bovine was Studied.
Materials and Methods
Materials
Soils Bio Dyne kit (Cat. No: 08-24-0000S) was used to amplify DNA fragments in qPCR.
Sampling
Twenty-six healthy male Holstein bovine were used in this study. Semen samples were taken using artificial vagina during early morning. Simultaneously, blood samples were collected from the caudal vein using gel-clot activator tubes. Rectal temperature of all bovines were taken immediately after blood sampling.
Blood and semen testosterone concentration
Serum and semen testosterone level were measured by AccuBind ELISA commercial kit (Monobind Inc. Lake Forest, USA, Cat. No: 3725-300). Immediately after blood collection, all tubes were puted in incubator 37˚C for 10 minutes to promotion clot formation. All tubes were centrifuged at3000 × g for 10 minute. Serums were collected and transfer to 2ml microtube and freezed at -70 untile hormone misurment. 0.5cc of semen samples were transfer to 1.5 ml microtube and freezed at -70 until hormone misurment.
Sperm concentration and viability
Collected semen was analyzed macroscopic and microscopic. Volume, color, density, contamination, concentration, viability ratio and motility were evaluated, high quality semen was used in this study.
DNA extraction, Primer design and qPCR
Total DNAs were extracted from sperm using salting-out protocol [24]. All chemical materials used for DNA extraction were obtained from Merck (Darmstadt, Germany). Concentration and purity of extracted DNAs were estimated by Nanodrop spectrophotometry absorption ratios at 260 nm and 260/280 nm respectively. The quality of extracted DNA was assessed by electrophoresis at 1% agarose-gel containing Ethidium Bromide. PAR gene was used as reference gene for normalization of expression data obtained from qPCR. The nucleotide sequences of genes, SRY (NCBI number: EU581861.1) and PLP (NCBI number: AJ009913.1) and PAR (NCBI number: AC234910.2) were obtained from NCBI (GenBank, National Center for Biotechnology Information). Primer pairs were designed using primer3Plus software and were shown in Table 1. The specificity of designed primers were evaluated using PrimerBLAST software of NCBI database.
Quantitative PCR was performed using SYBR Green super mix. The reactions consisted of 4μl SYBR Green PCR Master Mix (SYBR biopars, GUASNR, Iran), 0.5μl of each specific forward and reverse primers, 1μl of DNA, and 14 μl nuclease free water to a final volume of 20μl.
Statistical analysis
The data obtained from qPCR were analyzed according to the method of Livak&Schmittgen [25]. The mean Ct value was calculated for PAR and each of the two studied genes (PLP and SRY) and ΔCt value was determined for each gene in each sample using following formula:
ΔCt = Ct (target gene) - Ct (reference gene)
After calculation of ΔCt for all samples, the expression status of PLP and SRY genes relative to PAR was estimated using the following formula:Copy number of chromosome (X- or Y-chromosome) = 2-ΔΔCt = 2-(ΔCt (target gene) - ΔCt (PAR)
Finally, the ratio of PLP and SRY was considered as the ratio of X and Y chromosomes, respectively.All data were statistically analyzed using SAS computer software version 9.1 (SAS Institute Inc., Cary, NC, USA). The normality of data was tested by univariate procedure, and then the mean values were exposed to t-tests. Additionally, Pearson’s correlation coefficients between the ratio of PLP/SRY with some biological traits including blood and semen testosterone, semen concentration, age, rectum temperature, and viability of sperm were calculated using Corr in SAS software.
Results
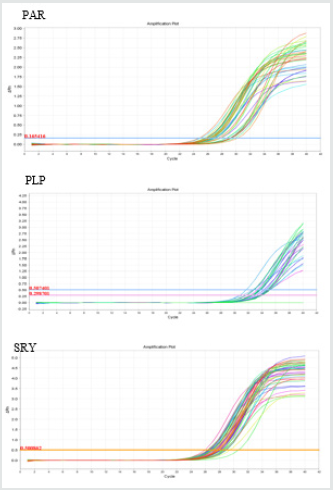
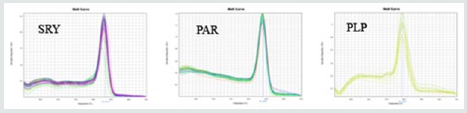
Electrophoresis of the extracted DNA had acceptable quality. Additionally, the least mean square values for the quantity of the extracted DNA were determined as 813.27±114.88 ng/μl, respectively. The amplification plots of PLP, SRY, and PAR genes plotted by Step One software (v.2.1) have been shown in Figure 1.The three studied genes provided a single peak in the melting curve. This implies on absence of primer-dimer formation during the reaction (Figure 2). The blood and semen testosterone concentrations are shown in Table 2. Wide variation was found in testosterone concentration of both blood and semen samples. The blood testosterone concentrations were reported ranging between 6- 12.5 ng⁄ml (Mean±SD: 9.45± 2.39 ng⁄ml) and also semen testosterone concentrations were reported ranging between 0.32- 4ng⁄ml (Mean±SD: 2.07± 1.59 ng⁄ml). There was a positive correlation between blood and semen testosterone concentration.A significant difference was found in the frequency of SRY- and PLPcarrying sperm of the sperm samples among the studied. The Y/X-chromosome bearing sperm was 1.75±0.44 (Mean±SD), ranging between 0.44-4.65 (Table 3). Y- Chromosome bearing sperm percentage was 53.7±12.5% (Mean±SD), Ranging between 18-82%. These data show a large variety in X and Y- chromosome bearing sperm population in different bovine semen.
Table 3: Compare mean 2−ΔcT SRY and PLP-carrying Sperm.

a,bValues with different superscripts within the same row differ significantly (p<0.05).
A strong correlation (0.98) was found between testosterone
concentration of blood and semen, meaning that cattle with
higher blood testosterone content have also higher testosterone
concentrations in their semen (Table 4). Furthermore, the correlation
coefficients of testosterone concentration of blood and semen and
the ratio of SRY-carrying sperm were 0.38 and 0.47, respectively,
meaning that bovine with higher testosterone concentrations
have significantly higher proportion of Y-chromosome bearing
sperm than those with lower blood and semen testosterone
contents. Additionally, the correlation coefficients of testosterone
concentration of blood and semen with the ratio of PLP-carrying
sperm were -0.67 and -0.60, respectively (Table 4).
A significant positive correlation (0.63, P<0.05) was detected
between the age of bovine and the ratio of SRY-carrying sperm
(Table 4). However, the correlation between age and the ratio of
PLP-carrying sperm was not statistically significant (-0.26, P>0.05)
(Table 5). Testosterone concentration of blood and semen was
positively correlated with age of bovine(0.72 and 0.79 respectively,
P<0.05). Rectum temperature was positively correlated to blood
testosterone concentration (0.41, P<0.05), but not correlated with
the ratio of SRY- and PLP-carrying sperm (-0.20, P>0.05) (Table 5).
The results of correlation analyses between different biological
characteristics of Holstein bovine have been summarized in Table
5. The correlation of rectum temperature with population of sperm
and sperm stimulation was 0.05 and -0.1, respectively, which were
not statistically significant (p>0.05).
Table 4: The correlation coefficient between PLP gene, SRY gene, blood testosterone, semen testosterone, age and rectum temperature among Holstein bovine.

*P < 0.05; **P< 0.01; and ns: non-significant (P > 0.05).
Table 5: The correlation coefficient between blood testosterone, semen testosterone, age, volume of semen, population and viability of sperm among Holstein bovine

*P< 0.05; **P< 0.01; and ns: non-significant (P > 0.05).
Discussion
There is a strong evidence of effect of environment and social
factors on sex ratio [26]. Several factors have been reported in bovine
that affect secondary sex ratio and also male calf is significantly
higher when Artificial Insemination (AI) used compared to natural
service [27,28]. Checa [29] reported that 50.02 ± 2.79% of sperm
bearing X-chromosome, but another study found that 44% of the
sperm carrier X-chromosome[30]. Lobel[31]examined 98 human
semen. They reported that 41.9-56.7% of the sperms bearing Y
chromosome. Another research reported similar data, 46.9-52.7%
Y-sperms in each ejaculation [32]. Madrid-Bury [33] reported
neither bovines nor semen didn’t have effect on Y-chromosome
bearing sperm percent or sex ratio of embryos produced in vitro
but the method of sperm preparation affected the primary sex ratio.
However, double swim-up sperm preparation method produced
differences in %Y- chromosome bearing sperm in some of sperm
fractions. They suggested that there are intrinsic differences in
capacitating of X and Y-bearing sperm that might be used to produce embryos of the desired sex in laboratory production of embryo.
However in two different studies on bovine semen, it became clear
that Y-chromosome bearing sperm population was between 24-
84% in each ejaculation[34,35]. The difference between previous
studies may be due to the breeds of the employed bulls (Holstein
and Galicia) or PCR techniques.
Wide variation was found in testosterone concentration in
both blood and semen samples. This variation may be related to
some factors such as individual difference, breed, and age. Although
the main source of testosterone in male is testicular tissue, the
concentration of semen testosterone in all studied animals was
much lower than blood. Lower levels of testosterone in semen
is due to transfer testosterone from blood in semen. Laydig cells
produce and release testosterone in blood, and semen testosterone
comes from blood[36].
Recently, studies have shown that offspring sex ratio is
significantly influenced by maternal dominance, a characteristic
which has been shown to be linked to testosterone in mothers[37,38].
Testosterone has been suggested to play an important role in the
viability of germ cells, probably by regulating a specific pathway for
apoptosis [39,40]. A lot of studies have been carried out to verify the
role of testosterone in alteration of sex ratio in different mammals
and our results were in agreement with these investigations. Helle
reported that a 1pg/ml increase in serum testosterone content of
rats could lead to 19% biased in sex ratio of offspring toward males.
They also showed that mothers with higher testosterone
produced more male offspring than those with low testosterone
content. James demonstrated that increase in blood testosterone
level in males can bias the sex ratio of offspring toward males.
Similarly, Shargal found that female ibexes (Capra Nubiana) with
higher fecal testosterone produced more male offspring. Grant
and Irwin and Grant focused on follicular testosterone, instead of
conventional serum and fecal testosterone, and demonstrated that
ova, developing in follicular fluid with high levels of testosterone,
were subsequently more likely to be fertilized by Y-chromosomebearing
spermatozoa, probably due to the differential stimulation
or viability. These authors proposed that there might be a critical
time, in which the follicular testosterone level affects the molecular
composition of zonapellucida and alters the susceptibility of
oocyte to be fertilized by a Y-bearing spermatozoon. According
to García-Herreros when the average testosterone level of bovine
follicular liquid exceeded 32.12 ng/ml, the probability of male birth
increased, while higher proportion of female birth was observed
when the follicular testosterone was around 23.98 ng/ml.
The important role of SRY in the viability of Y chromosome,
stimulation of apoptosis signaling pathway by PLP (due to
lipoprotein synthesis) and increasing of testosterone level, seems
to trigger the apoptosis of the X-chromosome bearing sperm
during the early stages of spermatogenesis. Additionally, some
other biological parameters of male Holstein cattle were found to
be influenced by testosterone concentration. Although, this is the
first study on the effects of paternal testosterone on offspring sex
ratio, a relatively large volume of studies on different mammalians
have demonstrated that mothers with higher blood, follicle or
fecal testosterone produce significantly more male offspring than
females with lower testosterone levels.
It has been reported that in terms of association between
the weather change and secondary sex ratio, by increasing of
1°Centigrades above the average value in the temperature of
weather during the week before fertilization, the probability of
male calf birth will increase about 1 % [41]. Evaporation rate
has the same effect on the birth of male calf. In addition, it has
been expressed that the birth of male and female in the hot and
cold weather is increased, respectively [42]. Perez-crespo[43,44]
reported that the birth of male can be increased by increasing the
temperature of environment and scrotum.
Conclusion
The results of the current study revealed a difference in percentages of Y and X -chromosome bearing sperm. A positive correlation between the frequency of SRY and testosterone was shown. Therefore, investigating the molecular mechanism involved in the effects of paternal testosterone on the ratio of its X/Y-chromosome bearing sperm may be an interesting subject for future studies.
Acknowledgments
The authors wish to acknowledge of Abbasabad Rearing and Breeding Station especially M. Jafari who has assisted in samples collection. We appreciate the staff of Laboratory of genetics, National Institute of Genetic Engineering and Biotechnology for technical assistance during this research.
References
- Delgado P, Lester T, Rorie R (2014) Variation among Beef Bulls in the Ratio of x- to Y-Chromosome Bearing Spermatozoa. Scientific Research 2(4): 69-75.
- Chandler JE, Steinholt-Chenevert HC, Adkinson RW, Moser EB (2007) Calving sex ratio as related to the predicted Y-chromosome-bearing spermatozoa ratio in bull ejaculates. Theriogenology 67(3): 563-571.
- Amadis A, Frana LM, Gandini V, Bornaghi K, Parati G, et al. (2015) Comparison between primary sex ratio in spermatozoa of bulls and secondary sex ratio in the deriving offspring. Theriogenology 83: 199-205.
- Seidel GE (2003) Economics of selecting for sex: the most important genetic trait. Theriogenology 59(2): 585-598.
- Seidel GE (2011) Sexing mammalian sperm-where do we go from here?. J Reprod Dev 58(5): 505-509.
- Miliaras D, Efthimiadis K, Meditesku S (2011) The sex ratio of fetal deaths and its potential relation with the decline of sex ratio at birth in Greece. Aristotle University Medical Journal 38(3): 27-35.
- Dominko T, First NL (1997) Timing of meiotic progression in bovine oocytes and its effect on early embryo development. Molecular Reproduction and Development 47(4): 456-467.
- James WH (2004) Further evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels around the time of conception. Human Reproduction 19(6): 1250-1256.
- Green MP, Spate LD, Parks TE, Kimura K, Murphy CN, et al. (2008) Nutritional skewing of conceptus sex in sheep: effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA). Reproduction Biology and Endocrinology 6(1): 1-11.
- Helle S, Helama S, Jokela J (2008) Temperature-related birth sex ratio bias in historical Sami: warm years bring more sons. Biology Letter 4(1): 60-62.
- Grant VJ (1994) Maternal dominance and the conception of sons. British Journal of Medical Psychology 67(4): 343-351.
- Malo AF, Martinez-Pastor F, Garcia-Gonzalez F, Garde J, Ballou JD, et al. (2017) A father effect explains sex-ratio bias. Proceedings of the Royal Society 284(861): 20171159.
- Gomendio M, Malo AF, Soler AJ, Fernandez-Santos MR, Esteso MC, et al. (2006) Male fertility and sex ratio at birth in red deer. Science 314: 1445–1447.
- Saragusty J, Hermes R, Hofer H, Bouts T, Goritz F, et al. (2012) Male pygmy hippopotamus influence offspring sex ratio. Nature Communication 3: 697.
- Douhard M, Festa-Bianchet M, Coltman DW, Pelletier F (2016) Paternal reproductive success drives sex allocation in a wild mammal. Evolution 70: 358–368.
- Shargal D, Shore L, Roteri N, Terkel A, Zorovsky Y, et al. (2008) Fecal testosterone is elevated in high ranking female ibexes (Capra nubiana) and associated with increased aggression and a preponderance of male offspring. Theriogenology, 69(6): 673-680.
- García-Herreros M, Bermejo-Álvarez P, Rizos D, Gutiérrez-Adán A, Fahey AG, et al. (2010) Intrafollicular testosterone concentration and sex ratio in individually cultured bovine embryos. Reproduction. Fertility and Development 22(3): 533-538.
- Parati K, Bongioni G, Aleandri R, Galli A (2006) Sex ratio determination in bovine semen: A new approach by quantitative real time PCR. Theriogenology 66(9): 2202-2209.
- Vatten LJ, Skjærven R (2004) Offspring sex and pregnancy outcome by length of gestation. Early human development 76(1): 47-54.
- Modi D, Shah C, Sachdeva G, Gadkar S, Bhartiya D, et al. (2005) Ontogeny and cellular localization of SRY transcripts in the human testes and its detection in spermatozoa. Reproduction 130(5): 603-613.
- Waters PD, Wallis MC, Graves JAM (2007) Mammalian sex-origin and evolution of the Y chromosome and SRY. Paper presented at the Seminars in cell & developmental biology 18(3): 389-400.
- Denny P, Swift S, Connor F, Ashworth A (1992) An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. The EMBO journal 11(10): 3705.
- Skoff R, Bessert D, Cerghet M, Franklin M, Rout U, et al. (2004) The myelin proteolipid protein gene modulates apoptosis in neural and non-neural tissues. Cell Death & Differentiation 11(12): 1247-1257.
- Kholghi M, Rostamzadeh F, Heidari F, Razmkabir M (2014) The comparesive of quality and quantity of extracted DNA from bovine sperm using salting out and phenol- chloroform method. Genetic in the 3rd millennium 12(1): 3254-3262.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 25(4): 402-408.
- Pavic D (2014) Sex ratio at birth in Croatia: update. Collegium Antropologicum 38(2): 559-563.
- Berry DP, Cromie AR (2007) Artificial insemination increases the probability of a male calf in dairy and beef cattle. Theriogenology 67(2): 346-352.
- Khan MS, Qureshi N, Chand A, Sultan R, Rafiullah I, et al. (2012) Effect of breeding method on calf sex and postpartum reproductive performance of cattle and buffaloes. Sarhad Journal of Agricolture 28: 469-476.
- Checa M, Dunner S, Canon J (2002) Prediction of X and Y chromosome content in bovine sperm by using DNA pools through capillary electrophoresis. Theriogenology 58(8): 1579-1586.
- Colley A, Buhr M, Golovan SP (2008) Single bovine sperm sex typing by amelogenin nested PCR. Theriogenology 70(6): 978-983.
- Lobel SM, Pomponio R, Mutter G (1993) The sex ratio of normal and manipulated human sperm quantitated by the polymerase chain reaction. Fertility and Sterility 59(2): 387-392.
- Mathews TJ, Hamilton BE (2005) Trend analysis of the sex ratio at birth in the United States. National vital statistics reports, 53(20), 1-17.
- Madrid-Bury N, Fernández R, Jiménez A, Pérez-Garnelo S, Moreira PN, et al. (2003) Effect of ejaculate, bull, and a double swim-up sperm processing method on sperm sex ratio. Zygote 11(03): 229-235.
- Chandler JE, Steinholt-Chenevert HC, Adkinson RW, Moser EB (1998) Sex ratio variation between ejaculates within sire evaluated by polymerase chain reaction, calving, and farrowing records. Journal of Dairy Science 81(7): 1855-1867.
- Chandler JE, Canal AM, Paul JB, Moser EB (2002) Collection frequency affects percent Y-chromosome bearing sperm; sperm head area and quality of bovine ejaculates. Theriogenology 57(4): 1327-1346.
- Douglas T, Carrell C, Matthew P (2010) Reproductive Endocrinology and Infertility. Springer-Verlag New York, USA, pp. 352-354.
- Grant VJ, Irwin R (2005) Follicular fluid steroid levels and subsequent sex of bovine embryos. Journal of Experimental Zoology Part A Comparative Experimental Biology 303(12): 1120-1125.
- Grant VJ, Irwin R, Standley N, Shelling A, Chamley L (2008) Sex of bovine embryos may be related to mothers' preovulatory follicular testosterone. Biology of reproduction 78(5): 812-815.
- Said TM, Paasch U, Glander HJ, Agarwal A (2004) Role of caspases in male infertility. Human Reproduction Update 10(1): 39-51.
- Ruwanpura SM, McLachlan RI, Meachem SJ (2010) Hormonal regulation of male germ cell development. Journal of Endocrinology 205(2): 117-131.
- Roche J, Lee J, Berry D (2006) Pre-conception energy balance and secondary sex ratio—partial support for the Trivers-Willard hypothesis in dairy cows. Journal of Dairy Science 89(6): 2119-2125.
- Boyle M, Schwanz LE, Hone J, Georges A (2014) How do climate-linked sex ratios and dispersal limit range boundaries?. BMC Ecology 14(1): 19-28.
- Pérez-Crespo M, Pintado B, Gutiérrez-Adán A (2008) Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Molecullar Reproduction and Development 75(1): 40-47.
- Berry DP, Kearney JF, Roche JR (2011) Evidence of genetic and maternal effects on secondary sex ratio in cattle. Theriogenology 75: 1039-1044.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...