
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4749
Research Article(ISSN: 2637-4749) 
A Novel Dendritic Cell Targeting PD-L1 Synthetic Vaccine as Potential Immunotherapy Strategy Elicits Therapeutic Antitumor Immunity Volume 4 - Issue 5
Maoping Huang1#, Wuyi Zeng1#, Jiayi Pan1#, Zixuan Fang1, Jiangtao Jia1, Rong Zhang1, Menghua He1, Jiashan He1, Xinyu Yang1, Bei Zhong1, Ailin Tao2, Jun Zeng1,2, Xueyan Zhang1,2*, Hui Liu1,2*
- 1The Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s hospital;School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou 510182, China
- 2The State Key Laboratory of Respiratory Disease, The Second Affiliated Hospital, Guangdong Provincial Key Laboratory of Allergy & Clinical Immunology, Guangzhou, China
- #These authors contributed equally to this work
Received: January 29th,2022; Published: February 11th, 2022
Corresponding author: Dr. Hui Liu. 195 DongFeng Xi Road, Guangzhou Medical University, Guangzhou510182, China. Email: liuhui806@gzhmu.edu.cn Or Dr. Xueyan Zhang. 195 DongFeng Xi Road, Guangzhou Medical University, Guangzhou510182, China. Email: zhangxy@gzhmu.edu.cn.
DOI: 10.32474/CDVS.2022.04.000199
Abstract
Tumor-specific Cytotoxic T Lymphocyte (CTL) can be activated in vivo by vaccination with Dendritic Cells (DCs), which is crucial for tumor control. Programmed Death Ligand 1 (PD-L1) is mainly expressed on the surface of tumor cells and Antigen-Presenting Cells (APCs) in various solid malignancies, implying an ideal choice for cancer immunotherapy and vaccine development. We recently reported a novel DC vaccination (PDL1-Vax) to actively induce anti-PD-L1 antibody and T cell responses capable of inhibiting PDL1+ tumor growth. In this study, we further tested whether vaccinations with DCs, loaded with another PD-L1 immunogen (PDL1- GMCSF), are also able to induce certain anti-PD-L1 immune responses. A synthetic vaccine composing the extracellular domain of PD-L1 linked to a T helper epitope sequence and a human GM-CSF is generated to improve the immunogenicity of tumor-specific antigen. Mouse pancreatic tumor generated by Panc02 cells is transfected with membrane-bound form of PD-L1 using retroviral platform so that tumor cells always express this antigen to home intratumoral DC. Vaccination with PDL1-GMCSF fusion protein loaded DCs yielded a prominent CD4+ Th production and elicited stronger protective T cell-dependent CTL immune response. This PDL1-GMCSF-containing vaccine showed significant therapeutic antitumor response and prolong the survival of tumor-bearing mice. These new findings expand previous and related knowledge and provide for the first time an efficient and promising strategy for tumor immunotherapy by PDL1-GMCSF protein-loaded DCs vaccination.
Keywords:CTL; dendritic cells; PDL1-GMCSF-containing vaccine; tumor Immunotherapy
Abbreviations:APCs: Antigen-Presenting Cells; CTL: Cytotoxic T Lymphocyte; DC: Dendritic Cell; DMEM: Dullbecco’s Modified Eagle’s Medium; FACS: Fluorescence-Activated Cell Sorting; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor; IFN: Interferon; IL: Interleukin; LPS: Lipopolysaccharide; mAb: Monoclonal Antibody; MHC: Major Histocompatibility Complex; PD-L1: Programmed Death Ligand 1; TAAs: Tumor-Associated Antigens; Th1: T helper type 1; TNF: Tumor Necrosis Factor
Introduction
Tumor cells always evade the immune system and tumor cells secrete chemicals that actively steer immune cells away from the tumor [1,2]. In order to bypass the immunosuppressive milieu and boost immune responses, an orchestrated approach is required encompassing mechanism which can target specific tumor-associated antigens for tumor treatment. Lots of trials have failed to show an improvement in survival when combined with other chemotherapeutic drugs [3]. Tumor cells can be recognized by tumor-specific T cells [4]. Tumor infiltration with cytotoxic T lymphocytes (CTLs) and T helper cells represents a favorable prognostic factor for patients with solid tumor [5,6]. This provides a strong rationale for the development of immunotherapy. Tumorspecific T cells can be activated by vaccination with dendritic cells (DCs) [7,8]. DCs are the most potent professional antigenpresenting cells (APCs) of the immune system, uniquely capable of stimulating tumor-specific CD4+ and CD8+ T cell immune responses leading to CTL tumor infiltration and tumor regression [9]. DCs are often pulsed with synthetic peptides from known tumor-associated antigens (TAAs), chemotherapy, or tumor lysate to generate an immune response [10]. Synthetic peptides corresponding to the epitopes recognized by T cells have also been used to enhance MHC class I binding and subsequently induce CTL response [11]. Pulsed DC vaccine has also been given along with standard chemotherapy to cancer patients [10,12]. Cytokines such as IL-2, IL- 12, granulocyte-macrophage colony-stimulating factor (GM-CSF), CEA and CD40 have been used as adjuvants to increase immune response [13,14]. Vaccines are the most clinically advanced part of tumor immunotherapy and the most encouraging results come from whole-cell, allogenic GM-CSF, DCs, and telomerase peptide vaccines [1,15]. Nevertheless, tumor cells can be rejected in vivo after vaccination with tumor antigen-loaded DCs [16]. Thus, combinations of vaccines with more appropriate trial designs are crucial for tumor immunotherapy.
Multiple T cell checkpoint molecules have been described to inhibit T cell activation [17,18], and the blockade of either of two of these inhibitory proteins, CTLA4 and PD1, has resulted in clinical benefit in several tumor types [19,20]. For some solid tumors such as the pancreatic cancer treatment, however, the neutralization of immune checkpoint receptors through CTLA-4 or PD-L1/ PD1 by itself proved to be ineffective [21], and new trails with nivolumab and pembrolizumab is ongoing in combinations with other vaccines or chemotherapy [1]. PD1 is a PD-L1 ligand that plays an important role in the inhibition of T cell-mediated immune response. Binding of PD1 to PD-L1 causes the exhaustion of effector T cells and immune escape of tumor cells. Several studies focused on pancreatic carcinoma have achieved similar results showing that PD-L1 expression in human pancreatic carcinoma tissues is associated with poor prognosis [22,23]. Since PD-L1 is rarely expressed on normal tissues but inducibly expressed on tumor site [24], it indicates that the selective expression of PD-L1 may have some association with clinical outcomes of the cancer patients and can be a selective target for antitumor therapy. A fusion protein of PD-L2 and Fc was shown to enhance the antitumor immune effect of GM-CSF-secreting whole-cell vaccine [25]. Most recently, we demonstrate, for the first time, the feasibility of DC vaccination (PDL1-Vax) to actively induce endogenous anti-PD-L1 antibody and T cell responses for cancer immunotherapy [26], providing a novel strategy could be used for cancer immunotherapy and immunoprevention. In this study, a synthetic fusion protein vaccine composing the human PD-L1 extracellular domain and human GM-CSF molecule employing the pET-21a/His system was further generated. We found such a PDL1-GMCSF protein-loaded DCs vaccine induced a potent antigen-specific antitumor CTL response in vivo and showed significantly therapeutic effect in mice tumor cells Panc02 stabling expressing human PD-L1. No obvious morphologic changes were visually observed from liver and kidney tissues immunized using PDL1-GMCSF-containing protein vaccine. These results may further provide a novel efficient strategy for tumor immunotherapy.
Materials and Methods
Mice and cell lines
Six- to eight-week-old female C57BL/6 mice were purchased from Jackson Laboratory and maintained locally. All animal procedures were approved by the Institutional Animal Care and Use Committee at Guangzhou Medical University. Murine pancreatic adenocarcinoma cell line Panc02 was originally established by Corbett et al. [27]. Both cell lines were maintained in DMEM supplemented with 10% (v/v) FBS, 1% L-glutamine, 1% sodium pyruvate, and 1% streptomycin-penicillin-neomycin solution (Biological Industries). Panc02 and MC38 stably expressing human PD-L1 were maintained in the media supplemented with 500 ng/ ml or 200 ng/ml puromycin (Invitrogen), respectively.
Protein production and purification
The recombinant vector pET-21a-PDL1-GMCSF was constructed by inserting a synthetic PD-L1-Th linker-GM-CSF into the vector pET-21a (Emd Millipore) by using NdeI/XhoI cloning sites. The recombinant plasmid was transformed into E. coli DH5α. The expressed fusion protein was purified by using a Ni-NTA agarose column according to the manufacturer’s instruction (Qiagen). The purified proteins were analyzed by using SDS-PAGE and Western Blot and then further dialyzed using a dialysis cassette following the manufacturer’s instructions (Thermo). The purified protein stocks were stored at -80°C for further study.
Retroviral-transduced tumor cell lines
To produce retroviral vectors, 293-T packaging cells were cultured in 100-mm culture dishes with DMEM medium (4.5 g/L glucose) supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% sodium pyruvate, and 1% A/A at 37°C in 5% CO2 and transfected with 1 ml TransfectagroTM Reduced serum medium (Corning) containing 10 μg PD-L1-retroviral vector plasmid, pCMVGag- Pol (10 μg) and pCMV-VSV-G (3.5 μg) and with the addition of polyethylenimine (PEI, 1 μg/μl) (the volume of PEI used was based on a 3:1 ratio of PEI (μg): total DNA (μg)). After overnight incubation, the medium was replaced with DMEM containing 5% FBS. 24 and 48 hours later, the culture medium containing recombinant retroviruses was collected and filtered through a 0.45 μm filter (EMD Millipore, Darmstadt, Germany). The non-concentrated harvested retroviruses were transduced into the exponentially growing tumor cell lines (Panc02 and MC-38) containing 8 ng/ml Polybrene. Then selected the positive PD-L1 expressing tumor cells with the addition of optimal final concentration of puromycin (1 mg/ml) as follows: Panc02 (4 μg/ml), MC-38 (2 μg/ml) and cultured those stably-expressing PD-L1 tumor cells and then harvested them for the further Flow Cytometry analysis.
Preparation of dendritic cells
C57BL/6 mouse bone marrow (BM)-derived dendritic cells (DCs) were prepared as described previously [26, 28]. In brief, mouse BM was flushed from limbs, passed through a nylon mesh, and depleted of red cells with ammonium chloride. After extensive wash with RPMI-1640, cells were cultured in RPMI-1640 supplemented with 10% FBS, mGM-CSF/ml (20 ng/ml) and recombinant mouse IL-4 (20 ng/ml; PeproTech). Nonadherent granulocytes were removed after 48 h of culture. Every other day, the supernatant was replaced with fresh media containing 20 ng/ml of rmGM-CSF and 20 ng/ml of rmIL-4. All cultures were incubated at 37 °C in 5% humidified CO2. Bacterial lipopolysaccharide (LPS; Sigma) was added at 100 ng/mL during the last 16 h for DC maturation. After 7 d of culture, >80% of the cells expressed characteristic DC-specific markers (CD40, CD80 and CD86) as determined by FACS.
DC immunization and tumor model
For preparation of antigen-pulsed DCs, BM-derived DCs were incubated overnight with 100 μg/ml of vaccine protein or control in culture media supplemented with the above cytokines. DCs were then pulsed again with 50μg/ml vaccine protein or control for an additional 2h. After washing with PBS three times, these antigen-pulsed DCs were used for immunization. C57BL/6 mice were randomly divided into two groups (5 mice per group) as follows: 1) PBS control, 2) hGM-CSF, and 3) PDL1-GMCSF. Mice were immunized via footpad injection two or three times at a one-week interval. To test efficacy of DC vaccines the immunized mice were subcutaneously inoculated with exponentially growing Panc02 or MC38 stably expressing human PD-L1 (5×104 cells) on the flanks one week after the last DC immunization. Tumor sizes were measured every three or four days with a caliper. Tumor volume was calculated as follows: (longest diameter) × (shortest diameter)2 [29].
Intracellular staining (ICS) and Flow cytometry analysis
Spleens were isolated from immunized C57BL/6 mice three days after the last DC administration. Prepared splenocytes (1×106 cells/well) were restimulated in U-bottom 96-well plates with 5 μg/mL freshly-prepared vaccine protein for 6 h in the presence of recombinant mouse IL-2 (20 ng/ml; PeproTech). 5 μg /mL Brefeldin A (eBioscience) was added to accumulate intracellular cytokines. After restimulation, the cells were firstly incubated with anti-mouse CD4, CD8, and CD11c antibodies for surface staining. Subsequently, intracellular staining for IL-2, IFN-γ, and Granzyme B were performed after these cells were fixed and permeabilized. Fixable viability dye was used to gate out dead cells. Data were collected on MMI FACS CANTO II (BD Biosciences, Los Angeles, CA, USA) and analyzed with Flow Jo software (Tree Star Inc., Ashland, OR USA). The antibodies used in this study were obtained from BD Biosciences (San Diego, CA, USA), including FITC anti-mouse CD4, APC anti-mouse CD8a, FITC anti-mouse CD40, APC anti-mouse CD80, FITC anti-mouse CD86, PE anti-mouse IL-2, eflour660 antimouse Granzyme B, and APC anti-mouse PD-L1 (CD274). Fixable viability dye was purchase from TONBO biosciences (San Diego, CA).
Enzyme-linked immunospot (Elispot) assay
ELISPOT assay was performed as described in our previous studies [30]. Briefly, Elispot plates (Millipore) were incubated with monoclonal anti-IFN-γ antibody (100 ng/well) overnight and then blocked with RPMI-1640 media supplemented with 10% FBS. Effector splenocyte (1×105, or 2×105) were co-cultured with protein (50 ng per well) in the coated plate for 24 h at 37 °C. After washing, biotinylated anti-mouse IFN-γ detection antibody was added to the wells, and then incubated for 2h. After another wash, HRP-conjugated avidin was added to the wells and incubated for 1 h at room temperature. Finally, freshly-prepared AEC Substrate Solution (Pierce) was added to the wells and then monitored development of spots. After spot development, the plate was rinsed thoroughly with ddH2O and allowed to dry. Spots were analyzed by a dissecting microscope and automated ELISPOT plate reader Zellnet Consulting, Inc. (New York).
Hematolxylin & Eosin (H&E) staining
Mouse liver and kidney were isolated from the above immunized groups and placed into isopentane for being fast frozen with liquid nitrogen. Tissue sections were performed according to the standard protocol. Briefly, the frozen tissues were cut (5μm thickness) at -20 °C and immediately transferred to a micro slide box kept on dry ice and stored at -80 °C. These slides were airdried, fixed with formalin, and then embedded with paraffin. H&E staining was completed at the pathological core in Guangzhou Medical University.
Statistical analysis
Statistical significance was determined using the t-test and One Way ANOVA test with Sigma Stat software. P< 0.05 was considered as a statistically significant difference. Regression plots were constructed using Sigma Plot software. All data were presented as the mean ±SEM and were representative of at least threeindependent experiments done in triplicate.
Results
Production of Recombinant PD-L1 Immunogens (PDL1- GMCSF)
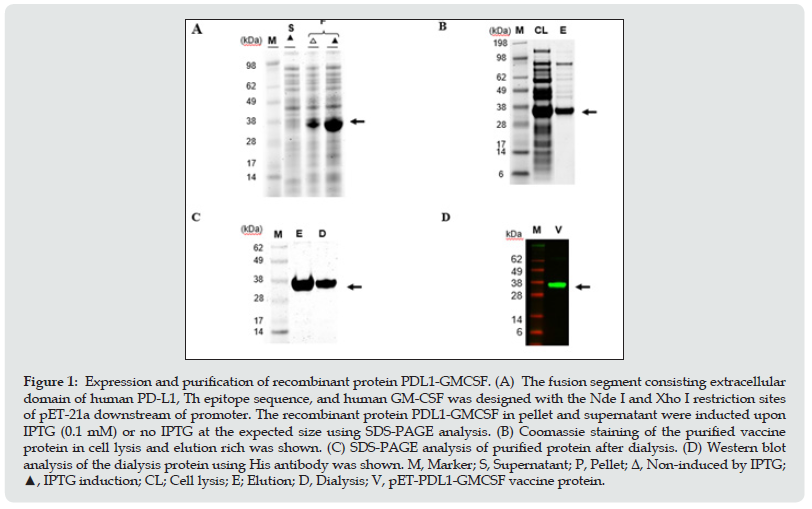
PD-L1 is mainly expressed on the surface of tumor cells and antigen-presenting cells in various solid malignancies such as pancreas and liver while rarely expressed on normal tissues [31,32], which means PD-L1 is a selective target for cancer immunotherapy and vaccine development. Cytokine GM-CSF is sable to mobilize lymphocytes and monocytes to tumor sites and has been used antigen delivery transit to increase immune response [33]. To generate a PD-L1 immunogen (PDL1-GMCSF), a fusion gene consisting of the extracellular domain of human PDL1 (aa 19–220) in-frame linked to a T helper epitope sequence and a human GM-CSF sequence was synthesized and cloned into Novagen pET21a expression vector to generate the expression vector pET-PDL1-GMCSF. For the expression of the recombinant protein (PDL1-GMCSF), this recombinant plasmid was then transformed into BL21 (E. coli) in the culture medium. After culture fermentation and isopropyl β-D-1-thiogalactopyranoside (IPTG) induction, the cells were collected, lysed, and examined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), where it was expressed in pellet with an expected molecular weight of approximate 34 kDa upon induction (Figure 1A, lane 3). Then the His-tagged protein vaccine was purified using Ni-NTA column under denaturing conditions, and the cell lysis and elute fractions was further analyzed by SDS-PAGE (Figure 1B). In order to create a physiological consistency, the elution rich fraction of purified protein was further dialyzed and then analyzed by SDS-PAGE (Figure 1C) and Western Blot using His antibody (Figure 1D). Using the purification procedures mentioned above, about 10 mg high purity recombinant PDL1-GMCSF vaccine protein was obtained from 1 L bacterial culture for further functional characterization.
Figure 1: Expression and purification of recombinant protein PDL1-GMCSF. (A) The fusion segment consisting extracellular domain of human PD-L1, Th epitope sequence, and human GM-CSF was designed with the Nde I and Xho I restriction sites of pET-21a downstream of promoter. The recombinant protein PDL1-GMCSF in pellet and supernatant were inducted upon IPTG (0.1 mM) or no IPTG at the expected size using SDS-PAGE analysis. (B) Coomassie staining of the purified vaccine protein in cell lysis and elution rich was shown. (C) SDS-PAGE analysis of purified protein after dialysis. (D) Western blot analysis of the dialysis protein using His antibody was shown. M, Marker; S, Supernatant; P, Pellet; Δ, Non-induced by IPTG; ▲, IPTG induction; CL; Cell lysis; E; Elution; D, Dialysis; V, pET-PDL1-GMCSF vaccine protein.
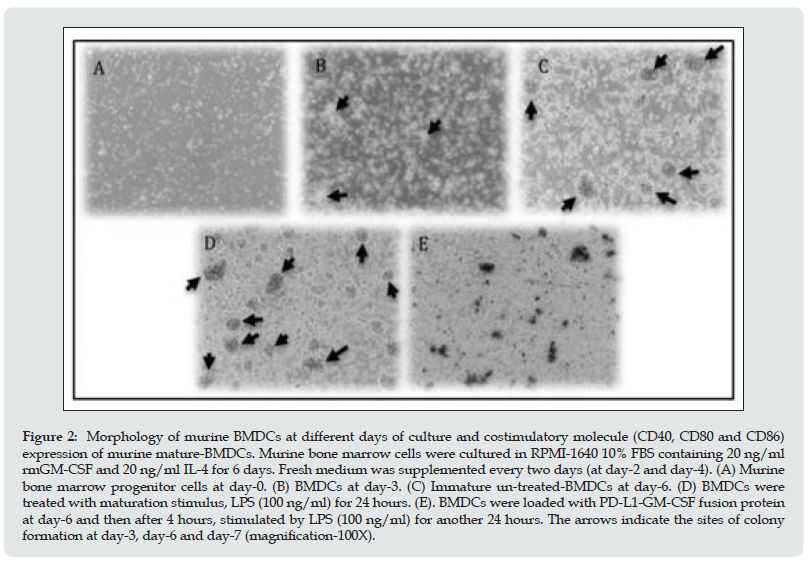
Maturation of the PDL1-GMCSF fusion protein-loaded DCs
Mouse bone marrow-derived DCs were prepared as we described previously [26,28]. On day 0, BM progenitor cells seeded into culture dishes showed a spherical morphology (Figure 2A). Cells were small, had defined cell membranes, and were in great health. On day 3, the initiation of colony formation happened at various sites with some aggregations of BMDCs and some cells were converted into adherent macrophages (Figure 2B). Then, on day 6, the immature BMDCs were obtained with the culture in the medium containing recombinant mouse GM-CSF (rmGM-CSF). Clusters of colonies of BMDCs were formed and amounts of floating and semi-adherent BMDCs were seen (Figure 2C). Subsequently, the cells were treated with maturation stimulus LPS (100 ng/ml) for 24 hours. Increased cell population and obviously huge clusters of colonies were seen. Simultaneously, the dendrites of cells were extended and more adherent macrophages were observed (Figure 2D and 2E (with the addition of fusion protein as well)). In addition, to detect the maturation of BMDCs, on day 6 of culture, the DCs were individually loaded with PDL1-GMCSF protein, PBS and LPS. After overnight stimulation, the expression levels of T-cell co-stimulatory molecules-CD40, CD80 and CD86, were determined by surface staining and further flow cytometry analysis. LPS was known as an essential inflammatory stimulus to induce DC maturation. In the meanwhile, the prominently increased release of T-cell costimulatory molecules, CD80 and CD86, was observed after LPS stimulation . The results show that the antigen uptake and DC maturation events have occurred during this culture and stimulation process..
Figure 2: Morphology of murine BMDCs at different days of culture and costimulatory molecule (CD40, CD80 and CD86) expression of murine mature-BMDCs. Murine bone marrow cells were cultured in RPMI-1640 10% FBS containing 20 ng/ml rmGM-CSF and 20 ng/ml IL-4 for 6 days. Fresh medium was supplemented every two days (at day-2 and day-4). (A) Murine bone marrow progenitor cells at day-0. (B) BMDCs at day-3. (C) Immature un-treated-BMDCs at day-6. (D) BMDCs were treated with maturation stimulus, LPS (100 ng/ml) for 24 hours. (E). BMDCs were loaded with PD-L1-GM-CSF fusion protein at day-6 and then after 4 hours, stimulated by LPS (100 ng/ml) for another 24 hours. The arrows indicate the sites of colony formation at day-3, day-6 and day-7 (magnification-100X).
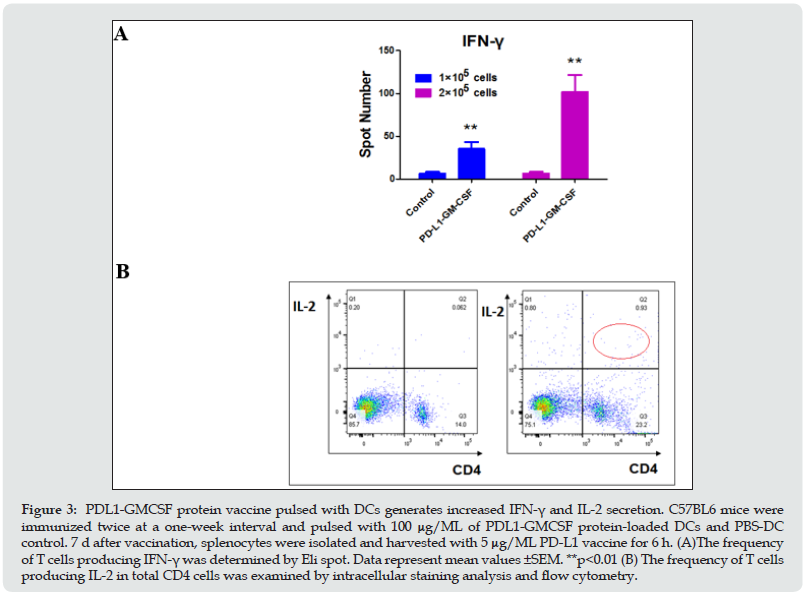
Higher level of Th1 cytokine secretion were observed in the mice splenocytes immunized with PDL1-GMCSF protein-pulsed DCs
To study whether the PDL1-GMCSF protein vaccine can induce efficient immune response, naive C57BL6 mice were immunized twice at a one-week interval with 100 μg/mL protein-loaded DCs and PBS-DCs. Three days later of the second vaccination, the spleens from the immunized mice were isolated and digested into singlecell suspensions, and then pulsed with 5 μg/mL vaccine protein again in a U-bottom 96-well plate for another 6h. The frequency of T cells producing IFN-γ was firstly determined by using Elispot. As expected, the T cells immunized with PDL1-GMCSF protein vaccine generated a significantly higher percentage of IFN-γ compared with cells immunized with PBS-DCs (Figure. 3A). An approximate 6- and 15-fold upregulation (respectively) of IFN-γ secretion was observed when coated 1×105 and 2×105 splenocytes in plate (Figure 3A). “The prominent increase of CD4+ and CD8+ population in PDL1-GMCSF protein-pulsed mice splenocyes was observed. The IL-2 producing CD4+ T cells were then determined and analyzed by intracellular staining and flow cytometry. As shown in Fig. 3B, the frequency of T cells producing IL-2 was induced a 15-fold increase (marked with red line) on total CD4+ cells, which is similar to extremely high IFN-γ induction”. These results clearly demonstrated that the PDL1- GMCSF protein vaccine elicit enhanced IFN-γ and IL-2 production and might generate good capability for CTL induction. These data perfectly indicate that this novel PDL1-GMCSF fusion proteinloaded DC vaccine elicits enhanced IFN-γ and IL-2 secretion from activated T lymphocytes, which would generate better potential to trigger CTL responses.
Figure 3: PDL1-GMCSF protein vaccine pulsed with DCs generates increased IFN-γ and IL-2 secretion. C57BL6 mice were immunized twice at a one-week interval and pulsed with 100 μg/ML of PDL1-GMCSF protein-loaded DCs and PBS-DC control. 7 d after vaccination, splenocytes were isolated and harvested with 5 μg/ML PD-L1 vaccine for 6 h. (A)The frequency of T cells producing IFN-γ was determined by Eli spot. Data represent mean values ±SEM. **p<0.01 (B) The frequency of T cells producing IL-2 in total CD4 cells was examined by intracellular staining analysis and flow cytometry.
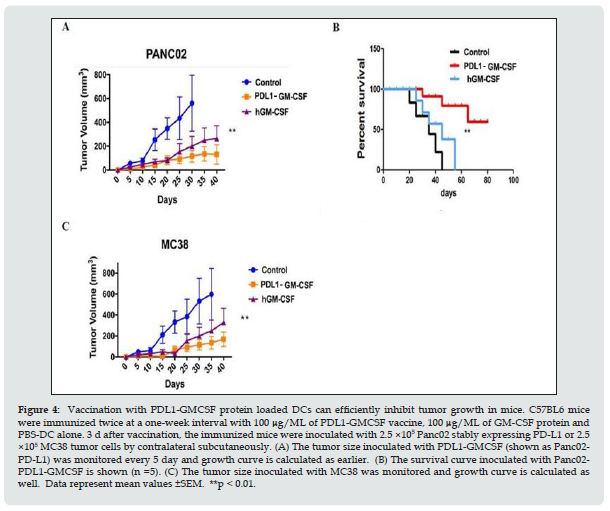
Therapeutic vaccination with DC targeting PDL1-GMCSF vaccine significantly inhibits tumor growth
Next, mouse cancer models have been used to test this fusion protein vaccine by conducting stable pancreatic cell line Panc02 expressing PD-L1 with retroviral transfection methods. The Panc02 tumor cell line stably expressing PD-L1 was performed as standard methods. Then 2.5 ×105 Panc02 tumor cells stably expressing PDL1 were injected contralateral subcutaneously into mice after 1 week of the second vaccination. After the tumor cell inoculation was established, the tumor growth was monitored every 5 day. As shown in Figure 4A, vaccination with PDL1-GMCSF group (shown as PDL1) more efficiently delayed tumor growth compared to both the GM-CSF vaccine and the PBS control group. Interestingly, the immunized mice from GM-CSF protein vaccine group also showed efficacy for the inhibition of tumor growth. Strikingly, the survival of tumor bearing mice was significantly improved by the PDL1-GMCSF protein vaccination, and 40% of treated mice survived at least 80 d (Figure 4B). The similar trend of tumor growth was observed for the same grouping of mice inoculated with MC38 tumor cells (2.5 ×105 tumor cells per mouse) (Figure 4C). Thus, these data imply that this fusion protein vaccine could be a more potent therapeutic vaccine compared with the conventional non-PD-L1 DC targeting protein vaccines.
Figure 4: Vaccination with PDL1-GMCSF protein loaded DCs can efficiently inhibit tumor growth in mice. C57BL6 mice were immunized twice at a one-week interval with 100 μg/ML of PDL1-GMCSF vaccine, 100 μg/ML of GM-CSF protein and PBS-DC alone. 3 d after vaccination, the immunized mice were inoculated with 2.5 ×105 Panc02 stably expressing PD-L1 or 2.5 ×105 MC38 tumor cells by contralateral subcutaneously. (A) The tumor size inoculated with PDL1-GMCSF (shown as Panc02- PD-L1) was monitored every 5 day and growth curve is calculated as earlier. (B) The survival curve inoculated with Panc02- PDL1-GMCSF is shown (n =5). (C) The tumor size inoculated with MC38 was monitored and growth curve is calculated as well. Data represent mean values ±SEM. **p < 0.01.
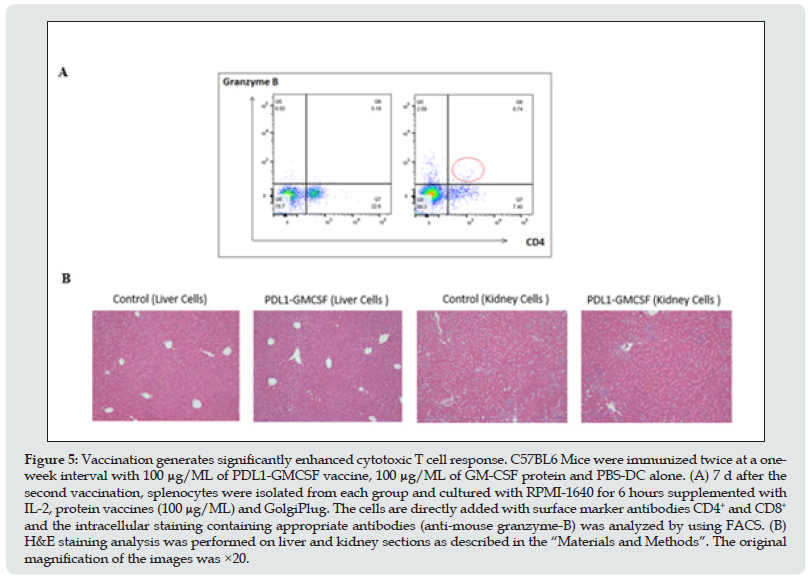
Vaccination generates significantly enhanced cytotoxic T cell response
Furthermore, to study the ability of the PDL1-GMCSF fusion protein-loaded DCs for the induction of specific anti-PD-L1 antibodies, the sera from the tails of immunized mice were collected and ELISA assays were applied to measure antibody levels. Levels of anti-PD-L1 antibodies (IgG) in sera of PDL1-GMCSF-loaded-DC immunized mice significantly increased (p<0.05) compared to other two immunized groups (hGM-CSF loaded-DC and PBS-DC/ control) and wild type (non-immunized with DCs) as well (data not shown). Once inside the host, DCs migrate into the lymphoid tissue and cause peptide reactive CTL responses [34], and then release cytotoxic effector molecules (such as perforins and granzymes), resulting in lysis of the recognized tumor cells [35,36]. Thus, naive C57BL6 mice were immunized with fusion protein-loaded DCs twice and spleen T cells were obtained 3 d after the second vaccination as earlier reported. Then the effector molecular granzymes B was tested to evaluate cytotoxic T cell response. Strikingly, the increased expression level of granzymes B was observed in immunized splenocytes using intracellular staining and FACS (Figure 5A), suggesting tumor cells lysis is priming and protective CTL response occurs. To further test possible adverse autoimmune pathology induced by immunization with PDL1-GMCSF-DCs. H&E staining on sections analysis of vital organs and tissues in the mice immunized with PDL1-GMCSF-DCs did not observe any significant pathologic inflammations (Figure 5B), In addition, some cells in the surface epithelium showed weak staining. Therefore, DC targeting PDL1- GMCSF vaccine induces efficient antitumor CTL responses and does not cause pathological toxicity in immunized mice.
Figure 5: Vaccination generates significantly enhanced cytotoxic T cell response. C57BL6 Mice were immunized twice at a oneweek interval with 100 μg/ML of PDL1-GMCSF vaccine, 100 μg/ML of GM-CSF protein and PBS-DC alone. (A) 7 d after the second vaccination, splenocytes were isolated from each group and cultured with RPMI-1640 for 6 hours supplemented with IL-2, protein vaccines (100 μg/ML) and GolgiPlug. The cells are directly added with surface marker antibodies CD4+ and CD8+ and the intracellular staining containing appropriate antibodies (anti-mouse granzyme-B) was analyzed by using FACS. (B) H&E staining analysis was performed on liver and kidney sections as described in the “Materials and Methods”. The original magnification of the images was ×20.
Discussion
Anticancer dendritic cells therapy currently uses in vitro propagation of the patient’s DC and pulsing with tumor antigens. However, clinical achievements are far from desirable. The greatest obstacles for DC-based therapy are the generation of a sufficient number of functionally active DC in tumors and effective DC pulsing with real time tumor antigens [37]. Any therapy with DC pulsed in vitro by a genetically static population of tumor antigens, followed by induction of tumor-specific cytotoxic T cells, could kill only a fraction of the tumor, because tumor cells continuously mutate by their very nature, unpredictably modifying tumor antigens [37]. Another theoretical barrier of currently implemented protocols with DC-based tumor therapy is that none of the protocols has incorporated an idea of intratumoral accumulation of injected DC. However, this event is logical for the induction of antitumor immunity and was detailed as such in the founding work [38]. A theoretical solution to the problem is let cancer cells orchestrate their own killing as long as they exist through making a stable transfection in situ of tumor cells with a membrane-bound forms of ligand and GM-CSF DNA [39,40], so that tumor cells always express membrane-bound forms of antigen and GM-CSF. Mouse cancer models have been used to test such approach as an anticancer therapy and experiments data showed that an increase in DC number (more than 10-fold) has a significant antitumor effect on pre-existing, poorly immunogenic tumors [41]. PD-L1 is mainly expressed on the surface of tumor cells and antigen-presenting cells in PC while rarely expressed on normal tissues, which makes PD-L1 is a selective target for tumor immunotherapy. In the current vaccine design, we have constructed and expressed a synthetic protein vaccine composing the extracellular domain of human PDL1 linked to human GM-CSF as an integral part of the protein vaccine to improve the immunogenicity. Tumor antigens fused with GMCSF were shown to activate ex vivo autologous peripheral-blood mononuclear cells, including APCs, and elicited antitumor effects in humans [42,43]. Therefore, such an inclusion of PDL1-GMCSF may offer an addition benefit for inducing CTL responses. Mouse pancreatic tumor generated by Panc02 cells is then transfected with PD-L1 peptide using retroviral platform so that tumor cells always express this antigen to home intratumoral DC. It found that this strategy could elicit therapeutic antitumor CTL response and significantly inhibit Panc02 tumor growth and prolong the survival of tumor-bearing mice. Our study provides a novel and easier method for generation of protein based DC-targeting tumor vaccine and an efficient strategy of immunotherapy. This may be especially useful when multiple neoantigens need to be targeted [44].
In an effort to enhance the amount of tumor antigen presented by DC, an alternative approach involved differentiating DC from monocytes in vitro, loading these with antigen and adjuvants and injecting these into patients as therapeutic vaccines. Our previous study have shown that the HSP-based Mage3 DNA vaccine can more effectively inhibit tumor growth by inducing both the innate immune responses and Mage3-specific adaptive immune responses via the Hsp-associated adjuvant function [45]. In the mouse, many other receptors have been exploited for the delivery of antigen and these studies have made a number of other salient points [46]. Cancer cells exploit the immune checkpoint to evade detection from T cells. The PD-L1-PD-1 axis plays important roles in limiting CTL responses and antitumor control. By blocking the PD-1/PDL1 pathway, cancer cells become exposed and the immune system becomes triggered to send out the alerting messages and launch a system-wide attack on cancer cells [47]. Indeed, significant anti-PD-L1 antibody response was observed in sera upon PD-L1 vaccination in this test (data not shown), implying it may partly block PD-L1/PD-1 signaling pathway. Thus, antibody-mediated cytotoxicity against tumors may be an important mechanism for tumor protection. However, whether the development of PD-L1- specific antibodies in mice immunized with PDL1-GMCSF-DCs is correlated with the levels of ADCC and CDC activities need to be determined. CTLs are critical effectors in antitumor immunity. To investigate the in vivo generation of a specific T cell responses elicited by this novel PDL1-GMCSF DC vaccine, antigen-specific CTL responses were tested in vitro. Effector cells obtained from mice immunized with this vaccine-pulsed DCs could effectively generated IFN-γ-secreting splenocytes, as indicated by ELISPOT assays. We also observed the increased level of IL-2 secretion from effector spleen cells, indicating a helper T cell response had been primed. The secretion of IFN-γ and IL-2 promote CTL development and delay hypersensitivity reactions, which will be favor of anticancer immunity.
To provide sufficient distribution of DC, pancreatic tumors generated by Panc02 PC cells is transfected with the membranebound form of PD-L1 using a retroviral platform, followed by inoculation of the transfected Panc02 PC cells back to the mice. This novel immunotherapeutic strategy could significantly inhibit Panc02 tumor growth and prolong the survival of tumor-bearing mice. We chose the Panc02 mouse tumor model because its poor immunogenicity resembles one of the characteristic features of human pancreatic carcinoma [48]. However, Panc02 cells can be recognised and killed by CTLs despite low levels of MHC class I expression, and immunotherapeutic approaches can be effective in this model [49]. Furthermore, it could prevent MC38 mice tumor growth. The results indicate that the use of DC as direct APCs could improve the potency of PDL1-GMCSF as a vaccine, which can include both strong T cell-mediated humoral and cellular anti- PD-L1 immunity for effective immunotherapy.
Till now, GVAX is the most promising vaccine immunotherapy especially in pancreas cancer [1]. Nevertheless, vaccine therapy has not produced the desirable results so far. Increasing evidence suggests that well established treatment strategies such as radiation, surgical debulking or chemotherapy may be successfully combined with immunotherapeutic approaches [50,51]. Thus, combination of this PDL1-GMCSF-containg vaccine with more appropriate trial designs may be promising approach of immunotherapy based on current information. Moreover, there are no apparent signs of toxicities and inflammations observed in the immunized mice, we intend to further investigate the potential autoimmune responses and toxicities induced by the PDL1-GMCSF DCs in immunized mice and cancer patients.
In summary, we have demonstrated that the PDL1-GM-CSF can stimulate robust T cell-mediated immunity. This vaccine could significantly inhibit tumor growth and prolong the survival of cancer-bearing animals. This work, together with our recent reported PDL1-Vax, lays out a new strategy to overcome the problem of immune tolerance by stable expressing PD-L1 tumor cell line for the development of effective tumor vaccines. This study also supports the potential benefits of developing new cancer immunotherapies that combine DC vaccination for application in humans. We propose that this type of immunotherapy may be applicable to pancreas, colon cancer, melanoma, leukemia, breast carcinoma, pulmonary cancer, prostatic carcinoma, and other types of tumors that highly express PD-L1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the members of SY Chen’s laboratory in University of Southern California for providing technical assistance and helpful suggestions. This work was supported by the startup foundation of Guangzhou Medical University (02-412-B205002- 1007053). This work was also supported by grants from the Sixth Affiliated Hospital of Guangzhou Medical University (No.1014155). This work was supported by the startup foundation of Guangzhou Medical University (02-412-B205002-1007053;JCXKJS2021B04).
References
- Kotteas E, Saif MW, Syrigos K(2016) Immunotherapy for pancreatic cancer. J Cancer Res Clin Oncol 142(8): 1795-1805.
- Colli ML, Hill JLE, Marroqui L, Chaffey J, Dos Santos RS, et al. (2018) PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine 36: 367-375.
- Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, et al. (2007) Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 56(9): 1275-1282.
- Ito M, Shichijo S, Tsuda N, Ochi M, Harashima N, et al. (2001) Molecular basis of T cell-mediated recognition of pancreatic cancer cells. Cancer Res 61(5): 2038-2046.
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, et al. (2004) CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28(1): e26-31.
- Hamid O, Ismail R, Puzanov I (2019). Intratumoral Immunotherapy-Update 2019. Oncologist 25(3): e423-e438.
- Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, et al. (2004) Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell-based immunotherapy. J Immunol 172(2): 1304-1310.
- Le Gall CM, Weiden J, Eggermont LJ, Figdor CG (2018) Dendritic cells in cancer immunotherapy. Nat Mater 17(6): 474-475.
- Melief CJ(2008) Cancer immunotherapy by dendritic cells. Immunity 29(3): 372-383.
- Amin M, Lockhart AC (2015) The potential role of immunotherapy to treat colorectal cancer. Expert Opin Investig Drugs 24(3): 329-344.
- Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, et al. (2007) Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother 30(7): 762-772.
- Wang D, Huang XF, Hong B, Song XT, Hu L, et al. (2018) Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight 3(3):e98368.
- Barth RJ Jr, Fisher DA, Wallace PK, Channon JY, Noelle RJ, et al. (2010) A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res 16(22): 5548-5556.
- Dougan M, Dranoff G, Dougan SK (2019) GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 50(4): 796-811.
- Miyake M, Hori S, Ohnishi S, Toritsuka M, Fujii T, et al. (2019) Supplementary granulocyte macrophage colony-stimulating factor to chemotherapy and programmed death-ligand 1 blockade decreases local recurrence after surgery in bladder cancer. Cancer Sci 110(10): 3315-3327.
- Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Benkoe T, et al. (2003) In vivo induction of dendritic cell-mediated cytotoxicity against allogeneic pancreatic carcinoma cells. Int J Oncol 22(3): 651-656.
- Chen L, Flies DB (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227-242.
- Kim HJ, Cantor H (2014) The path to reactivation of antitumor immunity and checkpoint immunotherapy. Cancer Immunol Res 2(10): 926-936.
- Sharma P, Allison JP. 2015. The future of immune checkpoint therapy. Science 348(6230): 56-61.
- Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4): 450-461.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455-2465.
- Geng L, Huang D, Liu J, Qian Y, Deng J, et al. (2008) B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol 134(9): 1021-1027.
- Wang L, Ma Q, Chen X, Guo K, Li J, et al.(2010) Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg 34(5): 1059-1065.
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, et al.(2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8): 793-800.
- Kojima M, Murata S, Mekata E, Takebayashi K, Jaffee EM, et al. (2014) Fusion protein of mutant B7-DC and Fc enhances the antitumor immune effect of GM-CSF-secreting whole-cell vaccine. J Immunother 37(3): 147-154.
- Chen J, Liu H, Jehng T, Li Y, Chen Z, et al. (2019) A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers (Basel) 11(2):1909.
- Cubas R, Zhang S, Li M, Chen C, Yao Q. (2011) Chimeric Trop2 virus-like particles: a potential immunotherapeutic approach against pancreatic cancer. J Immunother 34(3): 251-263.
- Shen L, Evel-Kabler K, Strube R, Chen SY (2004) Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol 22(12): 1546-1553.
- You Z, Hester J, Rollins L, Spagnoli GC, van der Bruggen P, et al. (2001) A retrogen strategy for presentation of an intracellular tumor antigen as an exogenous antigen by dendritic cells induces potent antitumor T helper and CTL responses. Cancer Res 61(1): 197-205.
- Huang XF, Ren W, Rollins L, Pittman P, Shah M, et al. (2003) A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res 63(21): 7321-7329.
- Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, et al. (2009) Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol 11(4): 394-402.
- Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe S, et al. (2015) Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer 88: 154-159.
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, et al. (2001) Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 19(1): 145-156.
- Proudfoot O, Pouniotis D, Sheng KC, Loveland BE, Pietersz GA (2007) Dendritic cell vaccination. Expert Rev Vaccines 6(4): 617-633.
- Anguille S, Lion E, Van den Bergh J, Van Acker HH, Willemen Y, et al. (2013) Interleukin-15 dendritic cells as vaccine candidates for cancer immunotherapy. Hum Vaccin Immunother 9(9): 1956-1961.
- Trapani JA, Smyth MJ (2002) Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2: 735-747.
- Subbotin VM (2014) Dendritic cell-based cancer immunotherapy: the stagnant approach and a theoretical solution. Drug Discov Today 19(7): 834-837.
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673): 245-252.
- Alsheikhly AR, Zweiri J, Walmesley AJ, Watson AJ, Christmas SE (2004) Both soluble and membrane-bound forms of Flt3 ligand enhance tumor immunity following "suicide" gene therapy in a murine colon carcinoma model. Cancer Immunol Immunother 53(11): 946-954.
- Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, et al. (1999) Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med 190(1): 125-133.
- Esche C, Subbotin VM, Maliszewski C, Lotze MT, Shurin MR (1998) FLT3 ligand administration inhibits tumor growth in murine melanoma and lymphoma. Cancer Res 58(3): 380-383.
- Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, et al. (2013) Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 81(6): 1297-1302.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5): 411-422.
- Delamarre L, Mellman I, Yadav M (2015) Cancer immunotherapy. Neo approaches to cancer vaccines. Science 348(6236): 760-761.
- Wang L, Rollins L, Gu Q, Chen SY, Huang XF (2009) A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the antitumor activity. Vaccine 28(2): 561-570.
- Caminschi I, Vremec D, Ahmet F, Lahoud MH, Villadangos JA, et al. (2012) Antibody responses initiated by Clec9A-bearing dendritic cells in normal and Batf3(-/-) mice. Mol Immunol 50(1-2): 9-17.
- Hammerich L, Binder A, Brody JD (2015) In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol 9(10): 1966-1981.
- Turnquist HR, Kohlgraf KG, McIlhaney MM, Mosley RL, Hollingsworth MA, et al. (2004) Tapasin decreases immune responsiveness to a model tumor antigen. J Clin Immunol 24(4): 462-470.
- Schmidt J, Ryschich E, Sievers E, Schmidt-Wolf IG, Buchler MW, et al. (2006) Telomerase-specific T-cells kill pancreatic tumor cells in vitro and in vivo. Cancer 106(4): 759-764.
- Broomfield S, Currie A, van der Most RG, Brown M, van Bruggen I, et al. (2005) Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res 65(17): 7580-7584.
- Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, et al. (2005) Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol 23(35): 8950-8958.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




