Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Research Article(ISSN: 2690-5760) 
Comorbidities and Impact on Mortality in Patients with Severe COVID-19 Infection in Third Wave of Epidemic in Myanmar Volume 4 - Issue 4
Khin Phyu Pyar1*, Aung Phyoe Kyaw2, Nyan Lin Maung2, Zar Ni Htet Aung2, Sai Aik Hla3, Soe Min Aung2, Win Kyaw Shwe3, Soe Win Hlaing2, Thein Tun Myint2, Kyaw Thet Maung2, Thurein Win2, Aung Thu2, Kyaw Zay Ya2, Myo Thant Kyaw2, Zay Phyo Aung2, Sit Min2, Min Aung Hein2, Myo Min Thant2, Min Lin Zaw OO2, Thein Soe Tun2, Kaung Myat2 and Tun Tun Win4
- 1Professor and Head/Senior Consultant Physician, Department of Medicine/ Department of Nephrology, Defence Services Medical Academy, No (1) Defence Services General Hospital (1000-Bedded), Myanmar
- 2Consultant Physician, No. (1) Defence Services General Hospital (1000-Bedded)
- 3Consultant Physician, No. (2) Defence Services General Hospital (1000-Bedded)
- 4Public Health Specialist, Myanmar
Received:June 01, 2022; Published: June 16, 2022
Corresponding author: Khin Phyu Pyar, Professor and Head/Senior Consultant Physician, Department of Medicine/ Department of Nephrology, Defence Services Medical Academy, No. (1) Defence Services General Hospital (1000-Bedded), Myanmar
DOI: 10.32474/JCCM.2022.04.000192
Abstract
Background: Coronavirus disease 2019 (COVID-19) has been a major threat to health around the world as it causes significant morbidity and mortality. Those with comorbidities may have severe form and fatal complications. The study aimed to assess the prevalent comorbidities and their impact on mortality in patients with severe COVID-19 infection.
Methods: A descriptive study was conducted in COVID-19 treatment centers in Myanmar- Yangon and Nay Pyi Taw, from June to October 2021. Data were collected by using standardized case report forms and then, a total of 404 severe COVID-19 infected patients (>18 years old) were included. The p value and odds ratio with a 95% confidence interval (CI) was used as a measure of association and the independent associated factors for severity of disease were investigated using logistic regression analysis.
Results: Among 404 patients, 258 (63.9%) were survivors; and 146 (36.1%) did not survive. Mean age was 62 years; most of them were male (60.6%). Old age, patients over 65 years old, was found to be one form of comorbidities; it was significantly related with mortality (odds ratio 0.47, 95% CI 0.31- 0.72; p < 0.001). Over eighty percent of them had comorbid diseases: hypertension (52.5%), diabetes mellitus (35.9%), cardiovascular disease (12.6%), obesity (10.1%) and chronic kidney disease (7.4%). Presence of comorbidity was significantly associated with increased mortality (odds ratio 0.28, 95% CI 0.14 – 0.55; p < 0.001). Significant association between mortality and comorbidities was detected in hypertension (odds ratio 0.49; 95% CI 0.32 – 0.74; p < 0.001), chronic kidney disease (odds ratio 0.40; 95% CI 0.19 – 0.86; p = 0.015) and malignancy (odds ratio 0.20; 95% CI 0.05 – 0.78; p = 0.014). The probable contributors for poor prognosis were neurological disease and chronic liver disease; nevertheless, they were not statistically significant (odds ratio 0.31; 95% CI 0.09 – 1.09; p = 0.054) (odds ratio 0.50; 95% CI 0.22 – 1.13; p = 0.09). Comorbid diseases which did not influence the outcome were diabetes mellitus (odds ratio 1.02; 95% CI 0.67 – 1.56; p = 0.931), chronic lung disease (odds ratio 0.10; 95% CI 0.30 – 2.42; p = 0.751), cardiovascular diseases (odds ratio 1.15; 95% CI 0.62 – 2.14; p = 0.656), pulmonary tuberculosis (odds ratio 1.38; 95% CI 0.48 – 3.99; p = 0.555), obesity (odds ratio 1.25; 95% CI 0.62 – 2.49; p = 0.533), current smoking (odds ratio 0.80; 95% CI 0.47 – 1.35; p = 0.399) and alcohol (odds ratio 1.02; 95% CI 0.52 – 1.99; p = 0.952).
Conclusions: Presence of even one comorbid disease did alter the outcome in patients with COVID-19 infection. Comorbid conditions like those with age older than 65 years, hypertension, chronic kidney disease and malignancy were related with mortality. Awareness of comorbidities on admission was essential for anti-viral therapy and anti-inflammatory treatments in order to reduce mortality. They should be first priority group in preventive measures, vaccination particularly in poor resource setting.
Keywords:COVID-19; Comorbidities; Mortality
Introduction
A novel human coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected humans in all age groups, of all ethnicities, both males and females while spreading through communities at an alarming rate since December 2019. The clinical manifestations range from a common cold to more severe fatal form- severe pneumonia, severe acute respiratory distress syndrome (ARDS), multi-organ failure, and even death. It is believed that COVID-19, in those with underlying health conditions or comorbidities, has an increasingly rapid and severe progression, often leading to death. Reported comorbidities in patients with COVID-19 disease were hypertension, diabetes mellitus, obesity, chronic kidney disease. Moreover, older patients were found to be the population at risks for acquiring COVID-19 disease as well as severity of disease and mortality. Because the COVID-19 deaths were mostly associated with at least one comorbidity, it should not be underestimated [1]. Some clinical manifestations like low oxygen saturation, falling conscious level, hypotension and severe chest radiographic involvement as well as laboratory parameters like low absolute lymphocyte count, high CRP, high ferritin and high D dimer were common poor indicators for mortality. Early identification of comorbidities was helpful in efficient patient management and possibly minimize the related mortality [1,2]. Preventive efforts should especially target those with comorbidities. This study analyzed the comorbid conditions on mortality rates in patients with severe COVID-19 infection.
Methods
Study design and participants
This descriptive study included 404 adult inpatients (≥18 years old) from February 2020 to August 2021. It was carried out at three purposively selected treatment centers, Mingaladon hospital (300-bedded), Phaung Gyi hospital (1500-bedded) and Nay Pyi Taw hospital (1000-bedded), which were designated for confirmed severe COVID-19 patients. Patients from Yangon Region were treated in Dagon hospital, Mingaladon hospital and Phaung Gyi hospital whereas those from Nay Pyi Taw region were hospitalized in Nay Pyi Taw hospital. All treatment centers have ICU facilities and treatment were given by junior physicians, supervised by senior consultant physicians with online meeting at least daily. All patients with severe SARS-CoV-2 infection, confirmed by a positive result on RT-PCR testing of a nasopharyngeal sample and WHO severity score ‘severe and critical form’, were included in this study. History taking, physical examination, chest radiograph and blood tests (ferritin, LDH, D-dimer and CRP), complete picture, liver enzymes, serum creatinine were done as according to hospital protocol. All patients received at least standard treatment according to Myanmar National guideline; remdesivir, glucocorticoids, antibiotics, prophylactic enoxaparin, oxygen, and nutritional support and supportive care. Follow up was done till discharge from hospital or death. The criteria for discharge were clinical improvement of symptoms, absence of fever for at least 48 hours, and nasopharyngeal swab sample negative for SARS-CoV-2 PCR. All medical records were kept confidential. Informed consent was taken from patients or from the patient’s legally authorized representative who could provide oral consent with appropriate documentation by the investigator. This study was approved by the hospital research and ethics committee of No. (1) Defence Services General Hospital (1000-Bedded) Mingaladon, Yangon.
Data collection
The clinical outcome was evaluated daily till discharge or death. Both clinical, radiological and laboratory data were collected in standardized proforma, and confidentiality was maintained. The data were checked by two medical officers and then, supervision, completeness, and consistency of collected data were performed by the principal investigator.
Operational definitions
Comorbidity was a presence of one or more additional medical conditions or diseases diagnosed by physicians. Day of symptom onset was the day when the initial symptom began such as runny nose, muscle ache, cough, sore throat, dyspnea, etc. The hospital outcome at the time of discharge from hospital (survival status) was either survivor or non-survivor. The discharge criteria were determined by attending physician.
Comorbid status was presence of one or more comorbid diseases like diabetes mellitus, hypertension, chronic kidney disease (early chronic kidney disease to end stage renal disease), cardiovascular disease (ischaemic heart disease, atrial fibrillation, heart failure), obesity (BMI more than 30), chronic lung disease (chronic obstructive airway disease, bronchial asthma), neurological disease (stroke, dementia), chronic liver disease (chronic liver disease with or without portal hypertension), malignancy (cancer, leukaemia, lymphoma). The comorbid associated group was having one or more comorbid disease and comorbid non-associated group did not have comorbid disease. Current smoking was current smokers irrespective of duration of smoking. Alcohol was both current drinker and those who stopped drinking two weeks ago. Immune status was defined as normal or immunocompromised. Immunocompromised status was those not having one of immunocompromised state transplant recipients, those on oral steroids for more than two weeks, those on immunosuppressants, systemic lupus erythematosus, diabetes mellitus, ESRD (eGFR < 30 ml/min), and, hematological malignancy. Normal immune status was those not having immunocompromised state.
Timing/duration of symptoms onset to admission (days) was time from first symptom to arrival at hospital.
Based on WHO severity score, the severity of COVID-19 was classified as mild, moderate, severe disease and critical disease. Mild form was symptomatic patients without evidence of viral pneumonia in CXR or hypoxia. Moderate form was confirmed patients with clinical signs of pneumonia (fever, cough, dyspnea, and fast breathing), CXR showed pneumonia and SpO2 on air is ≥ 95%. Severe form was confirmed patient with clinical signs of pneumonia (fever, cough, dyspnea, and fast breathing) adding one of the following: respiratory rate > 30 breaths per min, severe respiratory distress and SpO2 < 90% on room air. Critical form was confirmed COVID-19 patient with one or more of the followings: ARDS, sepsis, septic shock and acute thrombosis (pulmonary embolism, acute coronary syndrome, acute stroke).
Statistical analysis
Baseline clinical characteristics including age, gender, comorbid diseases, immune status and outcome were studied in this study. Continuous and categorial variables were present as mean (± SD) and number (%), respectively. We used Pearson chisquare test, X2 test and odd ratio with 95% confident interval level to detect association between clinical characteristics, risk factors and outcome among COVID-19 infected patients. To compare the mean clinical characteristics differences between survivors and non-survivors, student t-test was used. P value of less than 0.05 was considered statistically significant. Data entry was done into Microsoft Excel worksheet and statistical analyses were done using the SPSS software (version 22).
Results
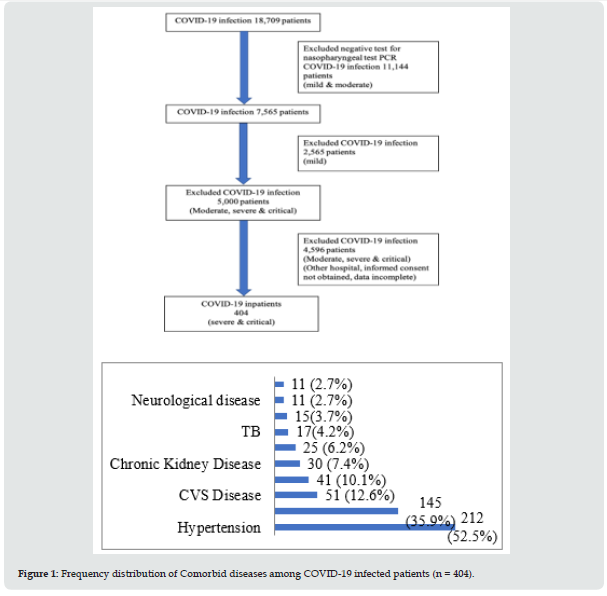
A total of 404 inpatients; 150 cases from Mingaladon hospital (300-bedded), 150 cases from Phaung Gyi hospital (1500-bedded) and 104 cases from Nay Pyi Taw hospital (1000-bedded) were included. Nearly two third of patients (258) were survivors; one third (146) did not make it. Table 1 shows baseline clinical characteristics and Table 2 reveals frequency distribution of clinical characteristics in groups. Mean age was 62 years; however, half of the cases were over 65 years. Most of them were male (60.6%). Over eighty percent of them had comorbid diseases: hypertension (212, 52.5%), diabetes mellitus (145, 35.9%), cardiovascular disease (51,12.6%), obesity (41, 10.1%) and chronic kidney disease (30, 7.4%). Malignancy and neurological diseases constituted less than 3%. It is illustrated in Figure 1.
Table 1: Frequency distribution of clinical characteristics among COVID-19 infected patients (n = 404).

Table 2: Associations between clinical characteristics and Outcomes among COVID-19 infected patients (n = 404).

Table 3: Associations between Comorbid diseases & risk factors and Outcomes among COVID-19 infected patients (n = 404).

Associations between comorbid diseases, risk factors and outcomes among COVID-19 in patients is shown in Table 3. Presence of comorbidity was significantly associated with increased mortality (odds ratio 0.28, 95% CI 0.14 – 0.55; p < 0.001). More than half of the patients had hypertension (212, 52.5%); it was related with mortality (odds ratio 0.49; 95% CI 0.32 – 0.74; p < 0.001). Chronic kidney disease was found in 30 patients (7.4%); however, it was significantly associated with death (odds ratio 0.40; 95% CI 0.19 – 0.86; p = 0.015). Malignancy was seen in less than 3% of patients, a strong predictor for non-survival (odds ratio 0.20; 95% CI 0.05 – 0.78; p = 0.014). The probable contributors for poor prognosis were neurological disease and chronic liver disease; nevertheless, they were not statistically significant (odds ratio 0.31; 95% CI 0.09 – 1.09; p = 0.054) (odds ratio 0.50; 95% CI 0.22 – 1.13; p = 0.09). Comorbid diseases which did not influence the outcome were diabetes mellitus (odds ratio 1.02; 95% CI 0.67 – 1.56; p = 0.931), chronic lung disease (odds ratio 0.10; 95% CI 0.29 – 2.42; p = 0.751), cardiovascular diseases (odds ratio 1.15; 95% CI 0.62 – 2.14; p = 0.656), pulmonary tuberculosis (odds ratio 1.38; 95% CI 0.48 – 3.99; p = 0.555), obesity (odds ratio 1.25; 95% CI 0.62 – 2.49; p = 0.533), current smoking (odds ratio 0.80; 95% CI 0.47 – 1.35; p = 0.399) and alcohol (odds ratio 1.02; 95% CI 0.52 – 1.99; p = 0.952).
Discussion
Coronavirus disease 2019 (COVID-19) has been a major threat to health around the world since end of 2019. It is believed that COVID-19, in those with underlying health conditions or comorbidities, has an increasingly rapid and severe progression, often leading to death. A hospital based descriptive study was conducted in COVID-19 treatment centers in Myanmar -Yangon and Nay Pyi Taw, Mingaladon Hospital, Phaung Gyi Hospital and Nay Pyi Taw Hospital from February 2020 to August 2021. Total 404 cases with confirmed severe COVID-19 infection were included; 258 (63.9%) survived and 146 (36.1%) did not survive. Over 80% of patients had comorbid diseases in this study. The majority of COVID-19 deaths had at least one comorbidity and it should be regarded as red flag sign [3,4]. Likewise, presence of comorbidity was significantly associated with increased mortality (odds ratio 0.28, 95% CI 0.14 – 0.55; p < 0.001) in this study. The study from Egypt revealed that the presence of one or more comorbidities worsened the survival rate of patients [5]. [6] found that patients with COVID-19 infection having comorbidities had prolonged hospital stay and higher mortality rate than those who did not have comorbidity. The prevalent comorbidity was hypertension 52.5%, diabetes mellitus 35.9%, cardiovascular disease 12.6%, obesity 10.1% and chronic kidney disease 7.4%.
Among the associated comorbid diseases, hypertension [odds ratio OR = 0.49; 95% CI 0.32 – 0.74; p < 0.001], chronic kidney disease (odds ratio 0.40; 95% CI 0.19 – 0.86; p = 0.015) and malignancy (OR: 0.20; 95% CI 0.05 – 0.78; p = 0.014) were significantly related with mortality; it was comparable with other studies from US, Germany and Canada [3,7-9]. Hypertension, diabetes mellitus and obesity were more likely to develop a more severe course and progression of the disease [4,10-13] and, in African study, they added renal disease, cancer and HIV infection [14,15]. Some comorbid disease like diabetes mellitus, chronic lung disease, cardiovascular diseases, pulmonary tuberculosis, obesity, current smoking and alcohol did not significantly influence the mortality in this study. “Diabetes mellitus was not significantly related with mortality in this study” was contrary to most of the findings [4,10-13]; the probable reasons were having good glycemic control in cases and relatively small sample size in this study.
Chronic kidney disease was found in 30 patients (7.4%); however, it was significantly associated with death (odds ratio 0.40; 95% CI 0.19 – 0.86; p = 0.015). They were on maintenance hemodialysis for end stage renal disease (ESRD). Several factors in cases with ESRD lead to mortality: low immunity, anemia, hypertension, diabetes mellitus, ischemic heart disease, hyperkalemia, low albumin, low absolute lymphocyte count and cardiomegaly in chest radiograph. Their total antibody level after vaccination was very low compared to those with normal renal function [14,15]. It confirmed previous reports [7,8,13]. Although malignancy was seen in less than 3% of patients (11 cases), it was a strong predictor for non-survival (odds ratio 0.20; 95% CI 0.05 – 0.78; p = 0.014). Malignancy was found to be a strong pointer for mortality in Canadian study, populationbased Cohort study involving 167,500 COVID-19 cases [9]. Though the number of severe cases having malignancy was small in this study, the mortality rate was the highest; nearly 70% of them succumbed. They were hematological malignancies and carcinoma of breast. [14] also highlighted the importance of associated malignancy in COVID-19 infection [13,15,16]. The probable contributors for poor prognosis were neurological disease and chronic liver disease though they were not statistically significant (odds ratio 0.31; 95% CI 0.09 – 1.09; p = 0.054) (odds ratio 0.50; 95% CI 0.22 – 1.13; p = 0.09). Eleven patients had neurological diseases: old cerebrovascular disease 5 cases, dementia 4 cases and parkinsonism 2 cases. They had several reasons favoring poor resolution of COVID-19 pneumonia:
a) Not having effective cough with sputum expectoration.
b) Difficulty in communication in patients with dementia.
c) Swallowing problems and
d) Silent aspiration.
Although neurological disease did not show statistically significant influence on outcome, they were very difficult for close nursing care particularly in cases with dementia. Dementia was a poor prognostic marker, reported by Canadian study (PLOS) and Korean study [16]. Half of the cases with cerebrovascular accident died; pre-COVID status of non-survivors of stroke cases were wheelchair bound though their swallowing was said to be normal. When they acquired severe COVID pneumonia, their coughing effort became weak. With the additive effect of poor chest expansion, they were vulnerable to mortality. It gave another evidence for stroke as an important factor [12,14,15,17]. Next comorbid disease having high mortality was chronic liver disease (odds ratio 0.50; 95% CI 0.22 – 1.13; p = 0.09); nearly half were non-survivors (43.75%). Patients with chronic liver disease had poor immune function causing severe pneumonia. The etiology was alcoholism and hepatitis B viral disease. They were in decompensated state of cirrhosis; and four cases had portal hypertension- ascites and splenomegaly. Chronic liver disease was rarely mentioned as increased risk of mortality in meta-analysis [14]; however, in the study done in Korea pointed out liver disease as poor prognostic factor [16].
Obesity was reported as a risk factor for mortality in several studies [14], and having multiple comorbidities and obesity showed a dose-response relationship [18]. Nevertheless, only one third of obese cases were fatal in this study. In the study done in Georgia, immunosuppression, hypertension, age over 65 years and morbid obesity were independent predictors of mortality [19]. However, systematic review by [13] found that obesity was not associated with mortality though it had high prevalence. Therefore, it was supported by this study; obesity was not associated with death (odds ratio 1.25; 95% CI 0.62 – 2.49; p = 0.533). In addition, the mortality rate of obese patients was more or less the same as that of cardiovascular disease, COPD and tuberculosis in this study. Chronic lung disease was not a risk factor for mortality (odds ratio 0.10; 95% CI 0.29 – 2.42; p = 0.751); it confirmed the finding “asthmatic patients were found to be at a reduced risk of COVID-19 hospitalization and severity in several large COVID-19 cohort studies” [20,21]. Although Systematic review & meta-analysis pointed out “comorbid respiratory disease was identified as the strongest risk factor for COVID-19 severity” [15,16], mortality rate of those with chronic respiratory diseases (COPD, Asthma) was 31.8% (7/22) in this study. It was emphasized that heart failure and atrial fibrillation were common cardiovascular markers for bad outcome [22], however it was not significantly related with (odds ratio 1.15; 95% CI 0.62 – 2.14; p = 0.656). Most of them had ischemic heart disease; some had stent, and some had coronary artery bypass graft. They had controlled heart failure.
Pulmonary tuberculosis due to Mycobacterium tuberculosis was mentioned as likely increased susceptibility to SARS-CoV-2 and increases COVID-19 severity in one study [23], however, it did not significantly influence the mortality in this study (OR: 1.38; 95% CI 0.48 – 3.99; p = 0.555). Because the majority of cases received antituberculous therapy for at least 2 months i.e., in sputum conversion state; and, they were not immunocompromised. In addition, their chest X-ray revealed mild patchy opacity in one or both upper zone; there was no evidence of collapse or consolidation. Effect of smoking on COVID-19 infection was negative. Smokers and previous smokers aged under 69 were at higher risk of COVID-19 infection; moreover, mortality rate of older smokers were twice than never smokers [24]. Smoking was independently associated with increased risk of mortality in patients with COVID-19 infection [25,26]. However, current smoking was not significantly related with mortality (odds ratio 0.80; 95% CI 0.47 – 1.35; p = 0.399). The possibility of association between severity of drinking and risk of COVID-19 infection as it causes general poor health [27]; effect of alcohol on liver may result in reduced immunity. Several reports mentioned increased prevalence of alcohol use during COVID-19 era particularly in lock-down period. Alcoholics were increased prevalent to COVID-19 infection [28]. Moreover, degree of alcohol drinking and mortality risks of COVID-19 was explained [27,29]. In this study, alcohol did not cause mortality significantly (odds ratio 1.02; 95% CI 0.52 – 1.99; p = 0.952). Patients over 65 years old (OR: 0.47, 95% CI 0.31– 0.72; p < 0.001) were found to be risk factor for severity and mortality; it was mentioned in the previous findings [4,16,30]. Report from one meta-analysis, patients with age over 50 years were associated with 15.4 folds significantly increased risk of mortality compared to patients with age younger than 50 year [14]. Regarding gender, male sex was not a risk factor for mortality in this study (OR: 1.12, 95% CI 0.73 – 1.69; p = 0.60); nevertheless, male sex was prone to severe COVID-19 infection and death in meta-analysis [14,31,32] mentioned that male sex had higher risk of COVID-19 infection; moreover, they were likely to have severe infection and death [2]. Those with immunocompromised state may have high fatality (OR: 0.72; 95% CI 0.44 – 1.18; p = 0.2); and they did benefit from convalescent plasma therapy [33]. It was clearly mentioned in other reports that immunocompromised state was a well-known risk factors for severe COVID-19 pneumonia and death.
Conclusion
Comorbidities such as age older than 65 years, hypertension, chronic kidney disease and malignancy were a strong risk factor for deaths among patients with COVID-19 infection. For prevention point of view, they must be in the list of vaccination priority groups particularly in poor resource setting. From therapeutic aspect, they should be in the first lists for hospital during pandemic period in order to reduce morbidity and mortality. They, themselves and their family members should be aware of risky nature. The treating physician should have awareness on importance of comorbidities in addition to clinical data and disease biomarkers to get efficient patient management and possibly minimize the related mortality.
Acknowledgements
The authors would like to thank all the candidates for giving informed consent to this study. The authors also acknowledged Prof Ko Ko Lwin, Prof Kyaw Zay Ya, Prof Myint Zaw, Prof Aung Myat Kyaw, Prof Khin Aung Tun and Dr Zaw Myo Han for administrative support, Professor Yu Aye Latt and team for intensive care, Professor Ohmar Hlaing for radiological support, and Professor Tin Moe Mya, Professor Tin Tin San, Professor Khine Khine Su and Dr Kyaw Wunna for laboratory support.
Declaration of Conflict of Interest
The authors declared no potential conflicts of interests with respect to authorship and publication of this article.
Ethical Approval
This study was approved by Hospital Research and Ethic Committee from Defence Services General Hospital (1000-Bedded) Mingaladon, Myanmar. Informed consent was also taken from each patient.
Funding
The authors received no financial support for publication of this article.
References
- Sharma J, Rajput R, Bhatia M, Arora P, Sood V (2021) Clinical Predictors of COVID-19 Severity and Mortality: A Perspective. Frontiers in Cellular and Infection Microbiology 11(1): 674277-674277.
- Li J, Huang DQ, Zou B, Yang H, Hui WZ, et al. (2021) Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. Journal of Medical Virology 93(3): 1449-1458.
- Antos A, Kwong ML, Balmorez T, Villanueva A, Murakami S (2021) Unusually High Risks of COVID-19 Mortality with Age-Related Comorbidities: An Adjusted Meta-Analysis Method to Improve the Risk Assessment of Mortality Using the Comorbid Mortality Data. Infectious Disease Reports 13(3): 700-711.
- Djaharuddin I, Munawwarah S, Nurulita A, Ilyas M, Tabri N A, et al. (2021) Comorbidities and mortality in COVID-19 patients. Gaceta Sanitaria (35 Suppl 2): S530-S532.
- Albadawy RM, Jadoon BA, Mogahed MM, Ibrahim ME, Essawy TS, et al. (2021) The Impact of Comorbidities on the Outcomes of Egyptian COVID-19 Patients: A Follow-Up Study. Journal of Environmental and Public Health 2021(1): 6662476-6662479.
- Fang H, Liu Q, Xi M, Xiong D, He J, et al. (2021) Impact of comorbidities on clinical prognosis in 1280 patients with different types of COVID-19. Journal of Investigative Medicine 69(1): 75-79.
- Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, et al. (2021) Causes of death and comorbidities in hospitalized patients with COVID-19. Scientific Reports 11(1): 4263-4265.
- Abayomi A, Osibogun A, Kanma Okafor O, Idris J, Bowale A, et al. (2021) Morbidity and mortality outcomes of COVID-19 patients with and without hypertension in Lagos, Nigeria: A retrospective cohort study. Global Health Research and Policy 6(1): 26-35.
- Ge E, Li Y, Wu S, Candido E, Wei X (2021) Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLOS ONE 16(10): e0258154-e0258159.
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, et al. (2020) Comorbidity and its Impact on Patients with COVID-19. SN Comprehensive Clinical Medicine 2(8): 1069-1076.
- Bae S, Kim SR, Kim MN, Shim WJ, Park SM (2021) Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 107(5): 373-380.
- Cheng S, Zhao Y, Wang F, Chen Y, Kaminga AC, et al. (2021) Comorbidities’ potential impacts on severe and non-severe patients with COVID-19: A systematic review and meta-analysis. Medicine 100(12): 24971-
- Ng Wern Hann, Tipih Thomas, Makoah Nigel A, Vermeulen Jan G, Goedhals Dominique, et al. (2021) Comorbidities in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. MBio 12(1): e03647-e03667.
- Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B (2021) Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology 64(1): 36-47.
- Zhou Y, Yang Q, Chi J, Dong B, Lv W, et al. (2020) Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. International Journal of Infectious Diseases 99(1): 47-56.
- Cho SI, Yoon S, Lee HJ (2021) Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Scientific Reports 11(1): 6375.
- Honardoost M, Janani L, Aghili R, Emami Z, Khamseh ME (2021) The Association between Presence of Comorbidities and COVID-19 Severity: A Systematic Review and Meta-Analysis. Cerebrovascular Diseases 50(2): 132-140.
- Mason KE, Maudsley G, McHale P, Pennington A, Day J, et al. (2021) Age-Adjusted Associations Between Comorbidity and Outcomes of COVID-19: A Review of the Evidence from the Early Stages of the Pandemic. Frontiers in Public Health 9(1): 584182-584182.
- Shah P, Owens J, Franklin J, Mehta A, Heymann W, et al. (2020) Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Annals of Medicine 52(7): 354-360.
- Green I, Merzon E, Vinker S, Golan Cohen A, Magen E (2021) COVID-19 Susceptibility in Bronchial Asthma. The Journal of Allergy and Clinical Immunology in Practice 9(2): 684-692.
- Caminati M, Vultaggio A, Matucci A, Senna G, Almerigogna F, et al. (2021) Asthma in a large COVID-19 cohort: Prevalence, features, and determinants of COVID-19 disease severity. Respiratory Medicine 176(1): 106261-106266.
- Phelps M, Christensen DM, Gerds T, Fosbøl E, Torp Pedersen C, et al. (2021) Cardiovascular comorbidities as predictors for severe COVID-19 infection or death. European Heart Journal - Quality of Care and Clinical Outcomes 7(2): 172-180.
- Chen Y, Wang Y, Fleming J, Yu Y, Gu Y, et al. (2020) Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. MedRxiv 2020.03.10.20033795.
- Prats Uribe A, Xie J, Prieto Alhambra D, Petersen I (2021) Smoking and COVID-19 Infection and Related Mortality: A Prospective Cohort Analysis of UK Biobank Data. Clinical Epidemiology 13(1): 357-365.
- Hou H, Li Y, Zhang P, Wu J, Shi L, et al. (2021) Smoking Is Independently Associated with an Increased Risk for COVID-19 Mortality: A Systematic Review and Meta-analysis Based on Adjusted Effect Estimates. Nicotine & Tobacco Research 23(11): 1947-1951.
- Umnuaypornlert A, Kanchanasurakit S, Lucero Prisno DEI, Saokaew S (2021) Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tob Induc Dis 19(1): 1-13.
- Dai XJ, Tan L, Ren L, Shao Y, Tao W, et al. (2022) COVID-19 Risk Appears to Vary Across Different Alcoholic Beverages. Frontiers in Nutrition 8(1): 772700-772705.
- Wang QQ, Kaelber DC, Xu R, Volkow ND (2021) COVID-19 risk and outcomes in patients with substance use disorders: Analyses from electronic health records in the United States. Molecular Psychiatry 26(1): 30-39.
- Huang BH, Inan Eroglu E, Shaban RZ, Hamer M, Britton A, et al. (2022) Alcohol intake and mortality risk of COVID-19, pneumonia, and other infectious diseases: An analysis of 437191 UK biobank participants. Preventive Medicine Reports 26(1): 101751-101754.
- Yang X, Yu Y, Xu J, Shu H, Xia J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8(5): 475-481.
- Chen J, Bai H, Liu J, Chen G, Liao Q, et al. (2020) Distinct Clinical Characteristics and Risk Factors for Mortality in Female Inpatients with Coronavirus Disease 2019 (COVID-19): A Sex-stratified, Large-scale Cohort Study in Wuhan, China. Clinical Infectious Diseases 71(12): 3188-3195.
- Liu R, Han H, Liu F, Lv Z, Wu K, et al. (2020) Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clinica Chimica Acta 505(1): 172-175.
- Pyar K P, Kyaw AP, Maung NL, Aung ZNH, Shan MA, et al. (2022) Efficacy of Convalescent Plasma Therapy (CPT) plus Remdesivir versus Standard Therapy (Remdesivir) In Patients with Severe or Critical COVID-19 Infection in Second and Third Wave of Epidemics in Myanmar: Non-Randomized Interventional Study. Suntext Review Virology 3(1): 1-20.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...