
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Research ArticleOpen Access 
Characterization of Libyan Cobra (Naja haje) Venom using Fluorescence and UV-Visible Spectroscopy Volume 2 - Issue 1
Inass A Sadaw1, Nisreen H Meiqa1, Salah M Bensabe1, Massaud Salem Maama2, Anton Herman3, Abdulathim A Alshousha4 and Abdul M Gba1*
- 1Department of Medicinal Chemistry, Faculty of Pharmacy, University of Tripoli, Libiya
- 2Zoology Department, University of Tripoli, Libiya
- 3Department of Biosciences, University of Salzburg, Austria
- 4Food and Drug Control Centre (LFDA), Tripoli, Libya
Received: March 11, 2020 Published: March 17, 2020
Corresponding author: Abdul M Gbaj, PhD, Associate Professor of Genetics and Biochemistry, Food and Drug Control Centre (LFDA), Tripoli, Libya
Abstract
Snake venoms act as a preparative to defend animals against predators and helps in immobilizing and digestion of prey. Venoms consist of a combination of enzymes and toxins, such as metalloproteases, phospholipase A2, L-amino acid oxidase and toxins, including cytotoxins and neurotoxins. In addition to their toxicity, venom components exhibit several pharmacological activities and can be used as templates for drug design. The Libyan cobra venom was studied using UV-visible and fluorescence spectroscopic techniques. The cobra protein main chain absorbs light in the region of 240-340nm. The aromatic sidechains of cobra venom contain tyrosine, tryptophan and phenylalanine which are responsible for the absorbance in this region. Cobra venom provides intrinsic fluorescence emissions due to excitation of tryptophan residues, with some contribution from phenylalanine and tyrosine emissions. In addition, di sulphide bridges contribute considerable absorption in this wavelength range. The main fluorescence obtained is due to tryptophan which has a wavelength of maximum absorption at 280 nm and an emission peak ranging from 310 to 350 nm. UV-visible absorption and fluorescence spectroscopic techniques are sensitive and rapid to study cobra venom in order to better comprehend the performance of this venom.
Keywords: Snakebite; Envenomation; Libyan Cobra; Protein Fluorescence
Introduction
Snake bites are crucial public health problem among many African countries including Libya, Tunisia, Egypt and Algeria [1,2]. Africa inhabits more than 400 snake species of which about 30 are extremely poisonous. These species which belong to four different families namely: Viperidae, Colubridae, Atracta spididae and Elapidae, were found to be responsible for most human fatalities, as reported by the World Health Organization (WHO) [3]. There are eight species of cobra snakes including Naja mossambica, Naja haje, Naja nigricollis, Naja siamensis, Naja kaouthia, Naja melanoleuca, Naja sputatrix and Pseudechis australis. The first seven species are cobras from the genus Naja and are found throughout Africa including Libya and Asia, while the king brown/mulga snake (P. australis) is indigenous to Australia (Cobras (Naja spp.)) [4-7].
Cobra and other snake venoms usually show absorption maxima between 275 and 280 nm which are caused by the absorbance of the two aromatic amino acids tryptophan (Trp) and tyrosine (Tyr) and, to a small extent, by the absorbance of cystine (i.e. of di sulphide bonds). The absorbances of Trp and Tyr depend on the microenvironment of their chromophores. They are slightly red-shifted when transferred from a polar to a non-polar environment [8-10]. Cobra venom contains three amino acids with intrinsic fluorescence properties, phenylalanine (Phe), tyrosine (Tyr) and tryptophan (Trp) but only tryptophan and tyrosine respond experimentally because their quantum yields are high enough to give a significant fluorescence signal. The three residues could be used to follow protein folding because their fluorescence properties (quantum yields) are sensitive to their environment which changes when the protein folds or unfolds [11-13]. The main aim of our study was to further characterize the Libyan cobra venom using spectroscopy techniques.
Materials and Methods
All experiments were conducted in Tris buffer (0.01M Tris, 0.1M NaCl at pH 7.4). Glass-distilled deionized water and analytical grade reagents were used throughout experiments. pH values of solutions were measured with a calibrated Jen way pH-meter model 3510 (Staffordshire, UK). All buffer solutions were filtered through Millipore filters (Millipore, UK) of 0.45 mm pore diameter.
Absorbance spectra
Absorbance spectra were measured with Analytic Jena Specord 200 Plus (1.4nm band width, scanning, dual beam, single cell holder spectrometer, London, UK) using quartz cells of 1.00 cm path length. UV-vis absorbance spectra were recorded in the 200-500 nm range and a spectral bandwidth of 3.0 nm. For the final spectrum baseline subtraction of the buffer solution was performed. The protein content of venom samples was determined by the spectrophotometric method of Markwell et al. [14]. Bovine serum albumin (BSA, Sigma) was used for standard assays.
Fluorescence spectra
Fluorescence emission and excitation spectra were measured using a Jasco FP-6200 spectrofluorometer (Tokyo, Japan) using fluorescence 4-sided quartz cuvettes of 1.00cm path length. The automatic shutter-on function was used to minimize photo bleaching of the sample. The selected wavelength chosen provided aggregate excitation of tryptophan and tyrosine residues. The emission spectrum was corrected for background fluorescence of the buffer.
Venoms
Libyan Cobra (Naja haje) venom was extracted by manual stimulation of the animal and obtained in liquid or semisolid form, respectively, from the Zoology Department, Faculty of Science, University of Tripoli (Libya) and stored at -20 °C until use. Venoms were added to 2ml of 0.01 M Tris, 0.1 M NaCl at pH 7.4.
Results and Discussion
Absorption spectra
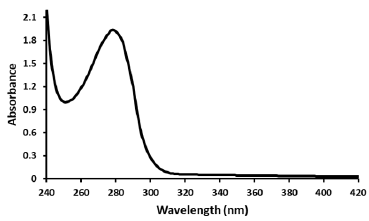
Absorption spectra of cobra venom (Figure 1) exhibit absorption maxima between 275 and 280nm which result from the absorbance of the two aromatic amino acids tryptophan (Trp) and tyrosine (Tyr) and to a small extent by the absorbance of cystine (i.e. of di sulphide bonds). The absorbances of Trp and Tyr were measured in 0.01 M Tris, 0.1 M NaCl at pH 7.4 and hence the spectra are specific to this microenvironment. Recently, it has been reported that absorption spectra of proteins are not primarily characterized by the ultraviolet region (185-320 nm) of the electromagnetic spectrum. but the peptide aggregates revealed absorption beyond 350 nm, caused by monomeric proteins lacking aromatic amino acids, di sulphide bonds, and active site prosthetic groups which were expected to remain optically silent beyond 250 nm [15,16]. It was also reported that UV-vis absorption profiles for monomeric proteins rich in charged amino acids spanning 250- 800 nm have opened a new label-free optical spectral window for probing biomolecular structure and interactions. By combining experimental and computational studies the authors suggested that the broad absorption profiles of these proteins arise from photo excited charge transfer (CT) transitions of spatially proximal charged amino acids such as lysine (Lys) and glutamate (Glu). The studies revealed that the tuned Lys-Glu dimer spectrum spans 150- 650 nm exhibiting five specific types of CT excitations with diverse and large spatial charge separation length scales of 2-10Å. These include inter-/intra-residue peptide backbone to peptide backbone (BB-CT) excitations spanning 160-210 nm, inter-/intra-residue peptide backbone to side chain (BS-CT) excitations spanning 160- 260 nm, and side chain to side chain (SS-CT) excitations, which show the broadest absorption range spanning 260-650 nm [17].
Figure 1: Ultraviolet-visible absorption spectrum of cobra venom (30 μg/ml) vs wavelength from 240-420 nm in 0.01 M Tris, 0.1 M NaCl at pH 7.4. The spectrum was corrected for small background fluorescence contributions from the buffer solution and was scaled to visualize the pure spectrum of the venom.
Fluorescence spectra
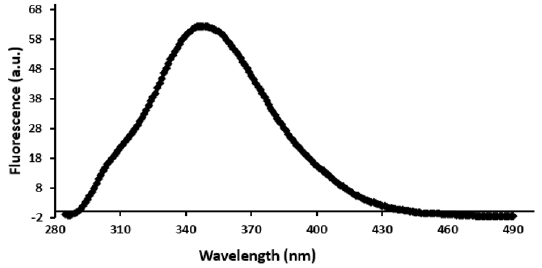
The fluorescence spectrum shows fluorescence intensity versus wavelength (Figure 2) of the Libyan cobra venom. The obtained fluorescence results from molecular rearrangements, excited-state reactions, ground state complex formation, collisional quenching and energy transfer. The fluorescence emission intensity as shown in Figure 2 exhibits a maximum emission at 350 nm suggesting that Trp residues activated by the excitation light moved from the buried hydrophobic environment into a relatively polar environment which is consistent with earlier reports [18].
Figure 2: Plot of fluorescence emission of Libyan Cobra (Naja haje) venom (30 μg/ml) vs wavelength from 285-490 nm using excitation of λ280 nm in 0.01 M Tris, 0.1 M NaCl at pH 7.4. Spectra were corrected for small background fluorescence contributions from the buffer solution and were scaled to visualize the shift. (a.u.: arbitrary units)
Similar results report an increase of intrinsic tryptophan fluorescence and a blue shift of the maximum emission wavelength upon addition of Mg ATP to Ars A ATPase (catalytic subunit of the pump protein), indicating the movement of Trp-159 into a less polar environment [19]. The fluorescence of Phospholipases A2 if excited at 280 nm is mainly due to the presence of a single tryptophan residue (Trp3) which is located on the surface of the enzyme molecule exposed to the environment [20].
As indicated in the literature the solvent polarity and the local environment have profound effects on the spectral emission properties of fluorophores. The effect of solvent polarity is an important determinant of the Stokes shift, which was clearly observed in our experiments. Emission spectra are easily measured resulting in numerous publications on emission spectra of fluorophores in different solvents or bound to proteins, membranes, DNA or RNA. One general use of solvent effects is to determine the polarity of the probe binding site at the macromolecule. This is accomplished by comparison of emission spectra and/or quantum yields if the fluorophore bound to the macromolecule or dissolved in solvents of different polarity. The effects of the environment on fluorescence spectra and quantum yields are complex and are due to several factors including: solvent polarity, viscosity, rate of solvent relaxation, probe conformational changes, rigidity of the local environment, internal charge transfer, proton transfer, excited state reactions, probe–probe interactions or changes in radiative and non-radiative decay rates. These multiple effects may offer chances to probe the local environment surrounding a fluorophore. However, environmental effects are usually complex and even solvent polarity cannot be described using a single theory. The Lippert-Ma taga equation partially explains the effect of solvent polarity but does not account for other effects such as hydrogen bonding to the fluorophore or internal charge transfer that depends on solvent polarity [21,22].
Conclusion
The UV-visible absorption and fluorescence spectroscope approaches are sensitive and fast techniques to help the possibilities in searching for natural or synthetic inhibitors for cobra snake venoms for therapeutic purposes. In future studies to investigate interactions and type of venom components considering candidates for these interactions could be performed. We expect that Libyan cobra venom will play an important part in the advancement of understanding snake poisoning (in the near future).
Conclusion
It is inferred in this study that the majority of cases of gestational syphilis were aged between 20 and 34 years old, the prevalent education level was that of incomplete elementary school, and the majority of pregnant women live in the urban area regarding the clinical classification, most cases were diagnosed in the primary phase. The number of cases of syphilis during pregnancy reported during the study interval in the city of Dourados-MS demonstrates the need to develop effective actions aimed at its control, as well as permanent health education for the population, since it is a disease totally avoidable, as long as there are prevention and treatment measures for the pregnant woman and the partner. Thus, it is necessary to continue studies in this area so that we can know the gaps found in public health that have not yet allowed to considerably reduce the cases of gestational syphilis and its possible problems for the fetus.
References
- Appiah B (2012) Snakebite neglect rampant in Africa. CMAJ 184(1): E27-E28.
- Harrison RA, Oluoch GO, Ainsworth S, Alsolaiss J, Bolton F, et al. (2017) Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl Trop Dis 11(10): e0005969.
- Appiah B (2012) Snakebite neglect rampant in Africa. CMAJ 184(1): E27-E28.
- Abdou F, Denshary EE, Shaaban E, Mohamed M (2017) Assessment of the neutralizing potency of antisera raised against native and gamma-irradiated Naja nigricollis (black-necked spitting cobra) venom in rabbits, concerning its cardiotoxic effect. Hum Exp Toxicol 36(12): 1335-1344.
- Abdel-Ghani LM, Rahmy TR, Tawfik MM, Kaziri I, Al Obaidi A, et al. (2019) Cytotoxicity of Nubein6.8 peptide isolated from the snake venom of Naja nubiae on melanoma and ovarian carcinoma cell lines. Toxicon 168: 22-31.
- Abdel Moneim AE, Ortiz F, Leonardo-Mendonca RC, Vergano-Villodres R, Guerrero-Martinez JA, et al (2015) Protective effects of melatonin against oxidative damage induced by Egyptian cobra (Naja haje) crude venom in rats. Acta Trop 143: 58-65.
- Kornhauser R, Isbister GK, O'Leary MA, Mirtschin P, Dunstan N, et al. (2013) Cross-neutralization of the neurotoxic effects of Egyptian cobra venom with commercial tiger snake antivenom. Basic Clin Pharmacol Toxicol 112(2): 138-143.
- McAninch SA, Morrissey RP, Rosen P, Meyer TA, Hessel MM, et al. (2019) Snake Eyes: Coral Snake Neurotoxicity Associated with Ocular Absorption of Venom and Successful Treatment with Exotic Antivenom. J Emerg Med 56(5): 519-522.
- Bae HD, Kim M, Lee J, Lee K (2018) Modified translationally controlled tumor protein-derived protein transduction domain enhances nasal delivery of exendin-4 as shown with insulin. Drug Deliv 25(1): 1579-1584.
- Bhattacharya S, Chakraborty M, Mukhopadhyay P, Kundu PP, Mishra R (2014) Viper and cobra venom neutralization by alginate coated multicomponent polyvalent antivenom administered by the oral route. PLoS Negl Trop Dis 8(8): e3039.
- Liu S, Sun MZ, Sun C, Zhao B, Greenaway FT, et al. (2008) A novel serine protease from the snake venom of Agkistrodon blomhoffii ussurensis. Toxicon 52(7): 760-768.
- Oda N, Yoshida M, Tanaka S, Kihara H, Ohno M (1986) Interactions of Trimeresurus flavoviridis phospholipase A2 and its N-terminal octapeptide-removed and p-bromophenacylated derivatives with acridine and anilinonaphthalene dyes. J Biochem 100(6): 1551-1560.
- Sun MZ, Liu S, Yang F, Greenaway FT, Xu Y (2009) A novel phospholipase A2 from Agkistrodon blomhoffii ussurensis venom: purification, proteomic, functional and structural characterizations. Biochimie 91(4): 558-567.
- Markwell MA, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87(1): 206-210.
- Prasad S, Mandal I, Singh S, Paul A, Mandal B, et al. (2017) Near UV-Visible electronic absorption originating from charged amino acids in a monomeric protein. Chem Sci 8(8): 5416-5433.
- Mandal I, Paul S, Venkatramani R (2018) Optical backbone-sidechain charge transfer transitions in proteins sensitive to secondary structure and modifications. Faraday Discuss 207(0): 115-135.
- Mandal I, Manna S, Venkatramani R (2019) UV-Visible Lysine-Glutamate Dimer Excitations in Protein Charge Transfer Spectra: TDDFT Descriptions Using an Optimally Tuned CAM-B3LYP Functional. J Phys Chem B 123(51): 10967-10979.
- Kenoth R, Simanshu DK, Kamlekar RK, Pike HM, Molotkovsky JG, et al. (2010) Structural determination and tryptophan fluorescence of heterokaryon incompatibility C2 protein (HET-C2), a fungal glycolipid transfer protein (GLTP), provide novel insights into glycolipid specificity and membrane interaction by the GLTP fold. J Biol Chem 285(17): 13066-13078.
- Zhou T, Rosen BP (1997) Tryptophan fluorescence reports nucleotide-induced conformational changes in a domain of the ArsA ATPase. J Biol Chem 272(32): 19731-19737.
- Cunningham TJ, Yao L, Lucena A (2008) Product inhibition of secreted phospholipase A2 may explain lysophosphatidylcholines' unexpected therapeutic properties. J Inflamm (Lond) 5: 17.
- Snitsarev V, Young MN, Miller RM, Rotella DP (2013) The spectral properties of (-)-epigallocatechin 3-O-gallate (EGCG) fluorescence in different solvents: Dependence on solvent polarity. PLoS One 8(11): e79834.
- Gong Y, Guo X, Wang S, Su H, Xia A, et al. (2007) Photophysical properties of photoactive molecules with conjugated push-pull structures. J Phys Chem A 111(26): 5806-5812.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




