Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5760
Research ArticleOpen Access 
Biochemical Manifestations of Sars-CoV-2 in Elderly Population (55 Years +): A Retrospective Cohort Study Volume 4 - Issue 4
Lelna Manu*, Imen Mbarek and Abdullah Al Naama
- Primary Health Care Corporation (PHCC), Qatar
Received: June 07, 2022 Published: June 27, 2022
Corresponding author: Lelna Manu, Primary Health Care Corporation (PHCC), Doha P.O. Box 26555, Qatar
DOI: 10.32474/JCCM.2022.04.000193
Abstract
Background: Several studies have demonstrated that age, comorbidities, and abnormalities in different clinical biomarkers can be important in understanding disease severity. Although clinical features of COVID-19 have been widely described, the assessment of alterations of the most common biochemical markers that are reported in patients with COVID-19 still has not been well established especially in the elderly population.
Methods: Here, we report the blood biochemical indicators of fifty-eight elderly patients (Age: 61.03±7.29) with COVID-19. Throatswab upper respiratory samples were obtained from patients, and real-time PCR was used to confirm SARS-CoV-2 infection. Then, biochemical parameters were categorized and analyzed according to these clinical characteristics. This retrospective study involved investigating the medical e-records of COVID-19 positive patients who presented to a COVID-19 centre in Qatar in July 2020.
Results: Of the 58 investigated patients, 58.62% were male and 41.38% were Female. 5.17% of the patients had low viral load (i.e., cycle threshold (Ct) ≥ 30) and 94.83% had a high viral load (ie; Ct < 30). Hypocalcemia and uremia affected 12.07% and hypochloremia affected 17.23% of the patients. 27.59% suffered from hyponatremia, 27.59 % had low creatinine, 24.14% had high levels of Alanine transaminase (ALT), 25.86% had high levels of aspartate transferase (AST), and 65.52% had a high level of C-reactive protein (CRP).
Conclusion: Elderly COVID-19 positive patients have been shown to have a disturbance in their biochemical parameters. Indeed, various biochemical parameters measured at baseline can provide useful prognostic information among COVID-19-affected patients. It can also help in the early identification of patients who merit aggressive institutional care, thereby potentially mitigating mortality.
Keywords: Geriatric Medicine; Geriatrics; Preventative Medicine; Public Health; Risk Factor; COVID-19
Introduction
The “Coronavirus Disease 2019” (COVID-19) pandemic started in late 2019 in Wuhan (Hubei Province, China) and has since spread to over 210 other countries/territories throughout the world. Many nations have implemented a number of public health measures since the beginning of the COVID-19 outbreak [1-5], including quarantine, isolation, travel restrictions, the closure of most nonessential activities, home isolation, and the closure of schools and institutions [3-9]. These initiatives are aimed at averting or, at the very least, mitigating the cost of the COVID-19 pandemic, as well as delaying and limiting the virus’s transmission [10-15]. According to recent studies, COVID-19 is a multi-systemic disease involving the cardiovascular, pulmonary, gastrointestinal, neurological, hematological, and immunological systems [15-18].
The biochemical profile of patients is a key factor in determining disease severity and prognosis [19]. Several investigations have looked at urea, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and salt levels in COVID-19 infections [19]. These variables have been linked not only to the severity of COVID-19 infection but also to the poorest outcomes in terms of in-hospital mortality [19]. Despite a large amount of literature on COVID-19, data on the epidemiological characteristics and clinical features of individuals of all ages and genders are scarce. Extensive research has been done, mostly on pediatric [20] and geriatric populations [21]. These studies rarely incorporate the patients’ laboratory findings and radiomics. Researchers frequently examine the relationship between demographic characteristics and underlying disorders and COVID-19 hospitalization [22-24].
Understanding the risk factors for non-mild diseases is important for risk classification and optimal treatment. Practitioners should know whether demographics (e.g., age, gender, ethnicity, country) are predictors of COVID-19 severity and outcomes. Asymptomatic or moderately symptomatic diseases are frequently overlooked since non-severe patients are not admitted to the hospital. This has an influence on risk assessment because a study cohort is not representative of the overall population. The same factor explains the variations in age-specific COVID-19 mortality rates reported in other countries (for example, China and Korea) [25]. Because the number of immunocompromised people in a population is linked to the age structure of that population, age appears to be a substantial risk factor for COVID-19 severity and consequences. A summary of studies on the age-related properties of COVID-19 is provided below.
Elderly COVID-19 patients are more likely to develop a severe illness [26]. Age-related comorbidities are the most likely cause of the increased mortality found in this age range [27-29]. However, doctors should avoid extrapolating age-related patterns from the group to the individual level. Otherwise, a patient may be classified as high or low risk based on their age rather than their real health status, which may result in incorrect risk assessment, inefficient resource allocation, and ineffective patient management. Thus, the purpose of this study was to access the biochemical profiles of elderly COVID-19 patients presenting to a COVID-19 facility in Qatar.
Methods
Data collection and participants
This was a retrospective data analysis cohort study that included individuals who were beyond the age of 55 based on their medical e-records. The participants in this study were referred to Rawdat Al Khail Health Site (RAK-HC), a COVID-19 testing center designated by the Ministry of Public Health in Doha, Qatar (MOPH). The study ran during the month of July 2020, and demographic and laboratory data for these individuals were collected from their medical e-records after the Primary Health Care Corporation (PHCC) research department anonymized and revealed patient details. The study was approved by the PHCC ethics committee under the reference number PHCC/DCR/2020/08/091. Of the 1054 COVID-19 positive patients seen at RAK-HC, 58 were over the age of 55 and had additional investigations, including a blood test, electrocardiogram (ECG), and a chest X-ray, as well as a thorough clinical assessment, to further assess the severity of COVID-19 infection, dictating further management of this group of patients. A complete blood count (CBC), a comprehensive metabolic panel (CMP), liver function tests (LFT), urea and electrolytes, random glucose, and a c-reactive protein (CRP) test were all performed on this patient group. We only looked at the biochemical parameters of this group in this investigation (ie; CMP, urea and electrolytes, LFT and CRP).
Results
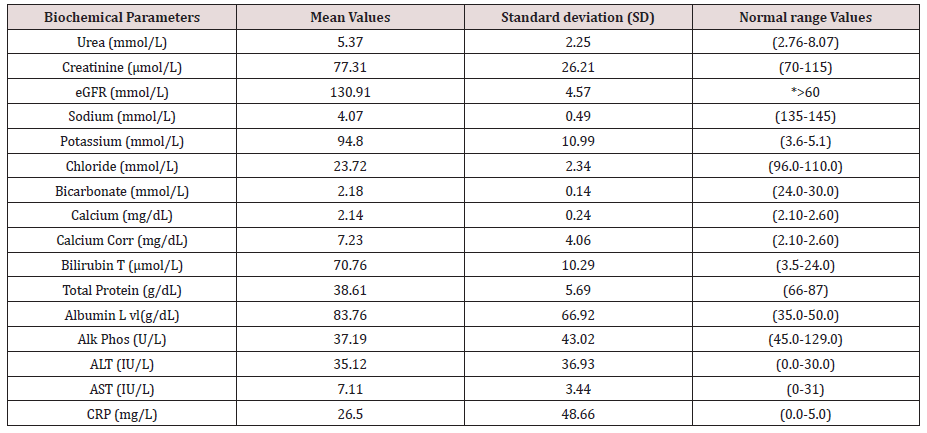
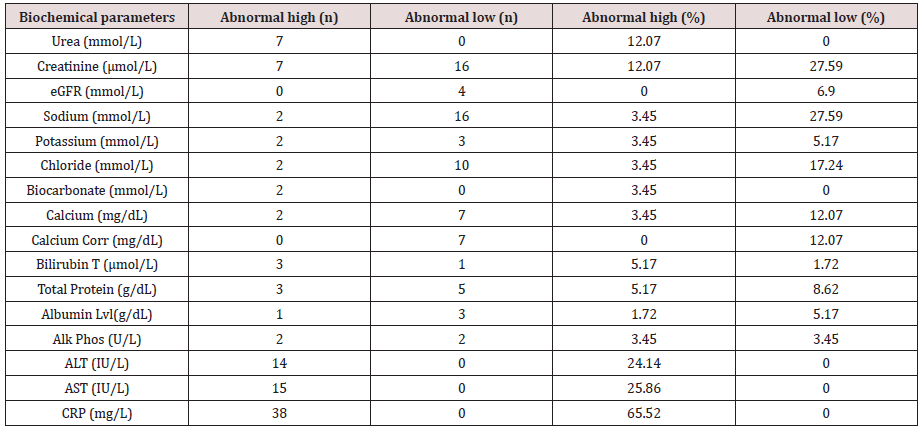
Amongst the 58 patients studied (58.62 percent Male and 41,38 percent Female). 5.17% of the patients had low viral load (i.e; cycle threshold (Ct) ≥ 30) and 94.83% had a high viral load (i.e; Ct < 30). Hypocalcemia and Uremia impacted 12.07 percent of the patients, while hypochloremia affected 17.23 percent (Figure 1, Tables 1 & 2). 27.59 percent had hyponatremia, 27.59 percent had percent creatinine, 24.14 percent had high levels of Alanine transaminase (ALT), 25.86 percent had high levels of Aspartate transferase (AST), and 65.52 percent had high levels of C-reactive protein (CRP) (Figure 1, Tables 1 & 2).
Figure 1: Comprehensive metabolic panel (CMP), liver function tests (LFT), urea and electrolytes, and a c-reactive protein test (CRP) abnormal rates (%) amongst the elderly COVID-19 patients.
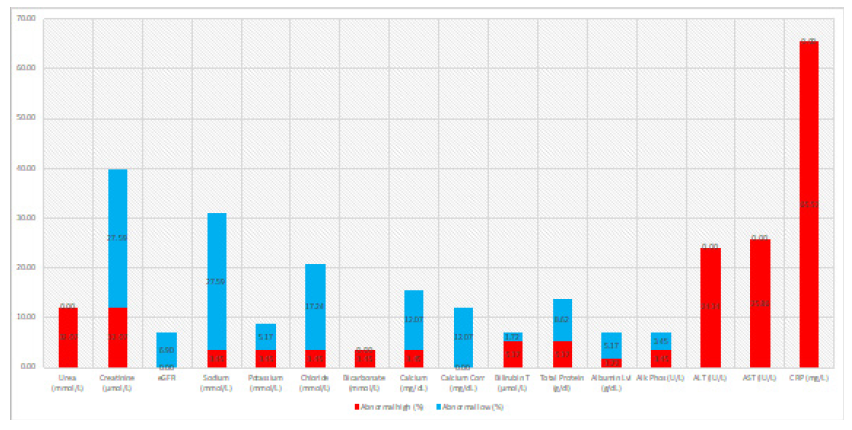
Table 1: Number and rates of abnormal biochemical parameters (i.e., Comprehensive metabolic panel (CMP), liver function tests (LFT), urea and electrolytes, and a c-reactive protein test (CRP)) amongst the elderly COVID-19 patients.
Discussion
The COVID-19 infection has become a global concern due to its pandemic features. There is currently insufficient understanding accessible, and breakthroughs in diagnostic technologies and treatment are urgently needed. Diagnostic procedures, such as RTPCR and antibody testing, are critical for epidemiology and early illness diagnosis [30]. Clinical, hematological, and biochemical markers must be monitored as part of COVID-19 patient treatment. Many anomalies were discovered in the biochemical parameters (i.e; comprehensive metabolic panel (CMP), liver function tests (LFT), urea and electrolytes, and a c-reactive protein test (CRP)) of COVID-19 older people in our study, which looked at those parameters.
Hypocalcemia and uremia affected 12.07 percent of our patients, while hypochloremia impacted 17.23 percent. 27.59 percent had hyponatremia, 27.59 percent had low creatinine, 24.14 percent had elevated Alanine transaminase (ALT), 25.86 percent had elevated aspartate transferase (AST), and 65.52 percent had elevated C-reactive protein (CRP). It is critical to categorize severity and outcome based on biochemical indicators. In this regard, bilirubin, ALT, AST, creatinine kinase (CK), C reactive protein (CRP), lactic dehydrogenase (LDH), cardiac troponin, ferritin, and fibrinogen have all been studied [31-33].
The identification of biochemical indicators accessible at the time of admission aids in the classification of COVID-19 severity and outcome, which is a valuable tool for healthcare practitioners. It can help with effective patient triage, personalizing interventions, monitoring clinical progress, and allocating appropriate resources at all levels of care to reduce morbidity and mortality. Severe COVID-19 infection causes a systemic inflammatory response and multiorgan dysfunction, which can impair renal function. COVID-19 individuals who have cofactors such as dehydration may have elevated blood urea levels. COVID-19 also infects renal tubule cells. What is more significant of them is being argued [34]. One of the COVID-19 infection symptoms is an abnormal renal profile. Increased urea levels were found to correspond with the severity of COVID-19 infection in a research involving 138 patients by Wang et al. [34]. Impaired renal function, according to Mahmoudi et al., is a major cause of death in COVID-19 patients [35]. In research by Li et al. [36], urea levels were found to be strongly associated with disease prognosis. In a meta-analysis [37], Henry et al. found a significant connection between urea and COVID-19 severity. According to Asghar et al., COVID-19 patients handled in the ICU had abnormal RFTs and poor outcomes [38]. The likelihood of multi-organ failure increases as severity increases.
Over a quarter of pneumonia patients have hyponatremia, which is related to severity and poor outcome [39]. Sodium anomalies in the COVID-19 setting have been very well investigated. Hyponatremia in COVID-19 infection is caused by the Syndrome of Inappropriate Antidiuretic Hormone (SIADH), which arises as a result of the production of inflammatory markers. In a study of 29 COVID-19 patients [40], Berni et al. discovered that hyponatremia, combined with elevated levels of interleukin-6 (IL-6), corresponded with the severity and outcome of COVID-19 infection [40]. Frontera et al. investigated the prevalence and consequences of hyponatremia (sodium 135 mmol/l) in 4654 COVID-19 infected patients. Hyponatremia affected 30% of these patients [16]. Hyponatremic patients also had an increased risk of mechanical breathing and a poorer outcome.
ALT and AST levels have been a topic of study in COVID-19 situations. In one investigation, both ALT and AST were found to be strongly linked with COVID-19 severity [39]. Only AST was found to be substantially linked with sickness severity in another investigation [34]. A significant association between ALT and AST and illness outcome has been established [36]. Henry et al. found a significant connection between ALT and AST and the severity of COVID-19 disease in a meta-analysis [37]. In a study conducted by Asghar et al. [38], AST was found to be substantially associated with severity. Our research found a link between COVID-19 severity and outcome and ALT and AST levels at admission. As a result, as the severity of COVID-19 infection grows, so do the signs of liver injury, which are associated with an increased risk of mortality. Weaker immunity and lower tolerance and response to infections in the elderly might lead to higher severity and a worse prognosis. According to Liu et al., the severity of the disease increases with age in COVID-19 individuals [41]. Age and comorbidities were linked to increased mortality in research by Yang et al. [42]. Wu et al. discovered that advanced age was associated with a greater fatality rate [43,44]. In a study conducted by Mahmoudi et al., elderly patients with decreased renal function had higher urea and creatinine levels [35]. An intriguing question arises here in that these molecular indicators may become disordered in other serious disorders. Urea levels have already been linked to mortality in non-COVID disorders. According to one study by Arihan et al. [45], increased urea levels (cut off >28 mg/dl) at admission have been linked to poor outcomes in patients admitted to ICU with non- COVID disease. According to Padhi et al., hyponatremia (Sodium levels 135) is also a predictor of lengthier ICU stay, longer duration of mechanical breathing, and greater mortality in ICU patients with disorders other than COVID-19 [46]. A study found that aberrant LFTs, with the exception of bilirubin, at the time of admission were strongly related to 30-day mortality in ICU settings. These, however, were not independent predictors of ICU 30-day mortality [47].
In terms of CRP, our findings revealed that 65.52 percent of the individuals studied had critically high levels of this protein. Indeed, it is unclear how CRP is linked to poorer survival in older people with COVID-19. First, CRP has been shown to be positively linked with lung lesions in the early stages of COVID-19 [48,49], suggesting that it may be a biomarker of disease severity [50,51]. Second, because CRP synthesis by hepatic cells is connected to IL- 6, CRP levels may represent IL-6 secretion caused by SARS-CoV-2 activation of monocytes, macrophages, and dendritic cells. The clinical significance stems from the fact that IL-6 is involved in the cytokine storm, which results in VEGF secretion and a decrease in E-cadherin expression, all of which contribute to increased vascular permeability, arterial hypotension, organ failures, and ARDS, with a risk of fatal outcome [52]. Third, the inflammatory condition demonstrated by elevated CRP levels may generate a prothrombin state, increasing the risk of arterial events such as stroke [53] or venous thromboembolic events such as pulmonary embolism [54].
Fourth, elevated CRP may be linked to an increased risk of death by producing hyper catabolism, which consumes respiratory muscle proteins, leaving less room for compensatory reactions in respiratory distress. Fifth, CRP levels may be a sign of older people’s pre-COVID-19 health condition, illustrating both the burden of chronic diseases (which are largely risk factors for severe COVID-19) and aging. Inflammaging is defined as the persistent activation of the innate immune system as we age, resulting in low-grade, chronic, regulated inflammation in older adults [56,57]. Inflammaging is characterized by an increase in the number of natural killer cells and an increase in the production of pro-inflammatory cytokines, particularly IL-6 and CRP [58]. This chronically inflamed state is harmful to health, adds to biological aging, and may explain the prevalence of severe and fatal COVID-19 forms in the older population.
Limitations
The current investigation has a number of flaws. The study only included COVID-19 older patients from Rawd et al. Khail Health Center, not all COVID-19 patients in Qatar, and data was collected only in July 2020, not during the full COVID-19 timeframe. Furthermore, no information on additional follow-up was provided in this analysis. The minimal number of patients evaluated is another key limitation of the present study.
Conclusion
The biochemical parameters of elderly COVID-19 positive patients have been proven to be significantly disturbed. Indeed, in COVID-19 patients, many biochemical markers assessed at baseline can provide important prognostic insights. It could also aid in the accurate diagnosis of patients requiring rigorous intensive care, potentially reducing fatalities.
Acknowledgments
We thank the PHCC research team for helping us provide the data needed for us to conduct the study.
Funding
This paper was not funded.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Author Contributions
a) Conception and design-Lelna MANU, Imen MBAREK, Abdullah AL NAAMA.
b) Analysis and interpretation of the data-Lelna MANU, Imen MBAREK.
c) Drafting of the paper-Lelna MANU, Imen MBAREK, Abdullah AL NAAMA.
d) Revising it critically for intellectual content-Abdullah AL NAAMA.
e) The final approval of the version to be published-Lelna MANU, Imen MBAREK, Abdullah AL NAAMA.
f) All authors agree to be accountable for all aspects of the work.
References
- (2020) WHO Virtual press conference on COVID-19.
- Varma A, Dergaa I, Ashkanani M, Musa S, Zidan M (2021) Analysis of Qatar’s successful public health policy in dealing with the Covid-19 pandemic. International Journal of Medical Reviews and Case Reports 5(2): 6-11.
- Varma A, AlDahnaim LA, Al Naama A, Vedasalam S, Mohammed AR, et al. (2021) Screening of Asymptomatic Passengers’ Departure from Qatar: A Retrospective Observational Study. Open Acc J Bio Sc 3(5): 1270-1275.
- Dergaa I, Varma A, Tabben M, Malik RA, Sheik S, et al. (2021) Organising football matches with spectators during the COVID-19 pandemic: What can we learn from the Amir Cup Football Final of Qatar 2020? A call for action. Biol Sport 38(4): 677-681.
- Musa S, Dergaa I, Abdulmalik MA, Ammar A, Chamari K, et al. (2021) BNT162b2 COVID-19 Vaccine Hesitancy among Parents of 4023 Young Adolescents (12–15 Years) in Qatar. Vaccines 9(9): 981-985.
- Abdulrahman H, Afify EM, Mohammed AS, Malik RA, Dergaa I (2021) Common Dermatological Complications of COVID 19: How Does it Affect the Skin?. Open Acc J Bio Sci 3(3): 1034-1038.
- Dergaa I, Abdelrahman H, Varma A, Yousfi N, Souissi A, et al. (2021) COVID-19 Vaccination, Herd Immunity and The Transition Toward Normalcy: Challenges with The Upcoming Sports Event. Annals of Applied Sport Science 9(3): e1032- e1042.
- Musa S, Dergaa I, Mansy O (2021) The puzzle of Autism in the time of COVID-19 pandemic: “Light it up Blue”. Psychology and Education 58(5): 1861-1873.
- Dergaa I, Musa S, Romdhani M, Amine Souissi, Mariam Ali Abdulmalik, et al. (2022) FIFA World Cup 2022: What can we learn from the inspiring Tokyo 2020 Olympic Games held in COVID-19 times?. Biol Sport 39(4): 1073-1080.
- Akbari HA, Pourabbas M, Yoosefi M, Briki W, Attaran S, et al. (2021) How physical activity behavior affected well-being, anxiety and sleep quality during COVID-19 restrictions in Iran. Eur Rev Med Pharmacol Sci 25(24): 7847-7857.
- Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, et al. (2021) Sleep quality and physical activity as predictors of mental wellbeing variance in older adults during COVID-19 lockdown: ECLB COVID-19 international online survey. International journal of environmental research and public health, 18(8): 4329-4335.
- Trabelsi K, Ammar A, Masmoudi L, Boukhris O, Chtourou H, et al. (2021) Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biology of Sport 38(4): 495-506.
- Varma A, Dergaa I, Mohammed AR, Abubaker M, Al Naama A, et al. (2021) Covid-19 and diabetes in primary care–How do hematological parameters present in this cohort?. Expert review of endocrinology & metabolism 16(3): 147-153.
- Dergaa Ismail, Muneer Abubaker, Amine Souissi, Abdul Rafi Mohammed, Amit Varma, et al. (2022) Age and clinical signs as predictors of COVID-19 symptoms and cycle threshold value. Libyan Journal of Medicine 17(1): 2010337-2010341.
- Dergaa I, Saad HB, Souissi A, Musa S, Abdulmalik MA, et al. (2022) Olympic Games in COVID-19 times: lessons learned with special focus on the upcoming FIFA World Cup Qatar 2022. British Journal of Sports Medicine 56(12): 654-656.
- Dergaa I, Musa S, Romdhani M, Amine Souissi, Mariam Ali Abdulmalik, et al. (2022) FIFA World Cup 2022: What can we learn from the inspiring Tokyo 2020 Olympic Games held in COVID-19 times?. Biol Sport 39(4): 1073-1080.
- Musa S, Elyamani R, Dergaa I (2022) COVID-19 and screen-based sedentary behaviour: Systematic review of digital screen time and metabolic syndrome in adolescents. PloS one 17(3): e0265560-e0265565.
- Musa S, Dergaa I, Tayebi SM (2022) Emergence of SARS-CoV-2 B. 1.1. 7 and the Future of Mega Sport Events: Is This the Tipping Point from Pandemic to Endemic?. Annals of Applied Sport Science 10(s1): e1114-e1119.
- Statsenko Y, Al Zahmi F, Habuza T, Almansoori TM, Smetanina D, et al. (2022) Impact of Age and Sex on COVID-19 Severity Assessed From Radiologic and Clinical Findings. Frontiers in cellular and infection microbiology 11(1): 777070-777075.
- Lu X, Zhang L, Du H, Zhang J, Li YY, et al. (2020) Sars-Cov-2 Infection in Children. N Engl J Med 382(17): 1663-1665.
- Lyu P, Liu X, Zhang R, Shi L, Gao J (2020) The Performance of Chest Ct in Evaluating the Clinical Severity of Covid-19 Pneumonia: Identifying Critical Cases Based on CT Characteristics. Invest Radiol 55(7): 412-421.
- Hsu HE, Ashe EM, Silverstein M, Hofman M, Lange SJ, et al. (2020) Race/Ethnicity, Underlying Medical Conditions, Homelessness, and Hospitalization Statusof Adult Patients With COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep 69(27): 864-869.
- Sapey E, Gallier S, Mainey C, Nightingale P, McNulty D, et al. (2020) Ethnicity and Risk of Death in Patients Hospitalised for Covid-19 Infection in the Uk: An Observational Cohort Study in an Urban Catchment Area. BMJ Open Respir Res 7(1): e000644-e000649.
- Deeb A, Khawaja K, Sakrani N, AlAkhras A, Al Mesabi A, et al. (2021) Impact of Ethnicity and Underlying Comorbidity on Covid-19 Inhospital Mortality: An Observational Study in Abu Dhabi, UAE. BioMed Res Int 2021(1): 6695707-6695711.
- Dudley JP, Lee NT (2020) Disparities in Age-Specific Morbidity and Mortality from Sars-Cov-2 in China and the Republic of Korea. Clin Infect Dis 71(15): 863-865.
- Liu K, Chen Y, Lin R, Han K (2020) Clinical Features of Covid-19 in Elderly Patients: A comparison with Young and Middle-Aged Patients. J Infect 80(6): e14-e18.
- Wang J, Zhu X, Xu Z, Yang G, Mao G, et al. (2020) Clinical and Ct Findings of Covid-19: Differences Among Three Age Groups. BMC Infect Dis 20(1): 434-445.
- Yang X, Yu Y, Xu J, Shu H, Liu H, et al. (2020) Clinical Course and Outcomes of Critically ill Patients With Sars-Cov-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir Med 8(5): 475-481.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. (2020) Clinical Course and Risk Factors for Mortality of Adult Inpatients with Covid-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 395(10229): 1054-1062.
- Duarte FB, Lemes RPG, Duarte IA, Duarte BA, Duarte JVA (2020) Hematological changes in Covid-19 infections. Revista da Associação Médica Brasileira 66(2): 99-99.
- Statsenko Y, Al Zahmi F, Habuza T, Neidl Van Gorkom K, Zaki N (2021) Prediction of COVID-19 severity using laboratory findings on admission: informative values, thresholds, ML model performance. BMJ open 11(2): e044500-e044504.
- Puah SH, Young BE, Chia PY, Ho VK, Loh J, et al. (2021) Clinical features and predictors of severity in COVID-19 patients with critical illness in Singapore. Scientific reports 11(1): 7477-7482.
- Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, et al. (2021) Predictors of COVID‐19 severity: a literature review. Reviews in medical virology 31(1): 1-10.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama 323(11): 1061-1069.
- Mahmoudi H, Alikhani MY, Taheri NM, Behzadi A (2020) Assessment of changes in blood urea and creatinine levels in patients with coronavirus disease 2019 (COVID-19). Research Square 1(1): 1-6.
- Li Q, Cao Y, Chen L, Wu D, Yu J, et al. (2020) Hematological features of persons with COVID-19. Leukemia 34(8): 2163-2172.
- Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G (2020) Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine (CCLM) 58(7): 1021-1028.
- Asghar MS, Kazmi SJH, Khan NA, Akram M, Khan SA, et al. (2020) Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: a single-center retrospective study in a tertiary care hospital of Karachi. Cureus 12(6): e8712-e8719.
- Frontera JA, Valdes E, Huang J, Lewis A, Lord AS, et al. (2020) Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Critical care medicine 48(12): e1211-e1217.
- Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, et al. (2020) Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together?. Journal of Endocrinological Investigation 43(8): 1137-1139.
- Liu Y, Mao B, Liang S, Yang JW, Lu HW, et al. (2020) Association between age and clinical characteristics and outcomes of COVID-19. European Respiratory Journal 55(5): 200111- 200115.
- Yang X, Yu Y, Xu J, Shu H, Liu H, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8(5): 475-481.
- Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13): 1239-1242.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Arihan O, Wernly B, Lichtenauer M, Franz M, Kabisch B, et al. (2018) Blood Urea Nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU. PloS one 13(1): e0191697-e0191699.
- Padhi R, Panda BN, Jagati S, Patra SC (2014) Hyponatremia in critically ill patients. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine 18(2): 83-87.
- Thomson SJ, Cowan ML, Johnston I, Musa S, Grounds M, Rahman TM (2009) ‘Liver function tests’ on the intensive care unit: a prospective, observational study. Intensive care medicine 35(8): 1406-1411.
- Wang L (2020) C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 50(4): 332-334.
- Tan C, Huang Y, Shi F, Tan K, Ma Q, et al. (2020) C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol 92(7): 856-862.
- Luo X, Zhou W, Yan X, Guo T, Wang B, et al. (2020) Prognostic Value of C-Reactive Protein in Patients with Coronavirus 2019. Clin Infect Dis 71(16): 2174-2179.
- Liu F, Li L, Xu M, Wu J, Luo D, et al. (2020) Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 127(1): 104370-104374.
- Moore JB, June CH (2020) Cytokine release syndrome in severe COVID-19. Science 368(6490): 473-474.
- Tsivgoulis G, Katsanos AH, Ornello R, Sacco S (2020) Ischemic Stroke Epidemiology During the COVID-19 Pandemic: Navigating Uncharted Waters with Changing Tides. Stroke 51(7): 1924-1926.
- Miesbach W, Makris M (2020) COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clin Appl Thromb Hemost 26(1): 1-5.
- Alzoughool F, Alanagreh L, Abumweis S, Atoum M (2021) Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin Hemorheol Microcirc 77(3): 311-322.
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, et al. (2000) Inflamm-aging. An evolutionary perspective on immuno senescence. Ann N Y Acad Sci 908(1): 244-254.
- Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1): S4-9.
- De Martinis M, Franceschi C, Monti D, Ginaldi L (2005) Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 579(10): 2035-2039.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...